
INOVELON 40 mg/ml SUSPENSION ORAL

Cómo usar INOVELON 40 mg/ml SUSPENSION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Inovelon40mg/ml suspensión oral
Rufinamida
Lea todo el prospecto detenidamente antes de empezar a tomar el medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. (Ver sección 4).
Contenido del prospecto:
- Qué es Inovelon y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Inovelon
- Cómo usar Inovelon
- Posibles efectos adversos
- Conservación de Inovelon
- Contenido del envase e información adicional
1. Qué es Inovelon y para qué se utiliza
Inovelon contiene un medicamento llamado rufinamida. Pertenece a un grupo de medicamentos llamados antiepilépticos, que se utilizan para tratar la epilepsia (una enfermedad que causas crisis convulsivas o ataques epilépticos).
Inovelon se utiliza con otros medicamentos para tratar las crisis convulsivas asociadas al síndrome de Lennox‑Gastaut en adultos, adolescentes y niños mayores de 1 año de edad. El síndrome de Lennox‑Gastaut es el nombre que recibe un grupo de epilepsias graves en las que se pueden presentar crisis repetidas de varios tipos.
Su médico le ha recetado Inovelon para reducir el número de crisis o ataques.
2. Qué necesita saber antes de empezar a tomar INOVELON
No tome Inovelon:
- si es alérgico a rufinamida o a los derivados triazólicos o a cualquiera de los demás componentes de Inovelon (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico:
- si tiene síndrome de QT corto congénito o historia familiar de este tipo de síndrome (alteración eléctrica del corazón), ya que el uso de rufinamida puede empeorarlo.
- si padece problemas hepáticos. La información sobre el uso de rufinamida en este grupo es limitada, por lo tanto puede ser necesario aumentar con más lentitud la dosis del medicamento. Si su enfermedad hepática es grave, el médico podrá decidir que Inovelon no es recomendable para usted.
- si desarrolla erupción cutánea o fiebre. Podrían ser signos de una reacción alérgica. Acuda al médico inmediatamente ya que muy ocasionalmente puede llegar a ser grave.
- si sufre un aumento en el número o severidad o duración de las crisis convulsivas, debe ponerse en contacto con su médico inmediatamente, si esto ocurre.
- si presenta dificultad para andar, movimientos anómalos, mareos o somnolencia, informe a su médico, si ocurre alguno de estos.
- si toma este medicamento y tiene pensamientos autolesivos o suicidas en algún momento, póngase en contacto con su médico o vaya al hospital de forma inmediata(ver sección 4).
Por favor, consulte con su médico, incluso si presentó estos efectos en algún momento en el pasado.
Niños
Inovelon no se debe utilizar en niños menores de 1 año porque no hay información suficiente sobre el uso en este grupo de edad.
Uso de Inovelonconotros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta. Si está utilizando los siguientes medicamentos: fenobarbital, fosfenitoína, fenitoína o primidona, puede necesitar que lo vigilen cuidadosamente durante dos semanas al comienzo o al final del tratamiento con rufinamida, o después de cualquier cambio importante en la dosis. Puede ser necesario cambiar la dosis de los otros medicamentos ya que pueden ser menos eficaces cuando se administran con rufinamida.
Antiepilépticos e Inovelon
Si el médico le receta o le recomienda un tratamiento adicional para la epilepsia (p. ej., valproato), debe informarle que toma Inovelon ya que puede ser necesario ajustarle la dosis.
La toma de valproato al mismo tiempo que rufinamida en niños y adultos dará lugar a niveles altos de rufinamida en la sangre. Informe a su médico si está tomando valproato, ya que es posible que deba ajustarle la dosis de Inovelon.
Informe a su médico si utiliza anticonceptivos orales/hormonales es decir, “la píldora”. Inovelon puede hacer que la píldora no sea eficaz para prevenir el embarazo. Por lo tanto, se recomienda que utilice además otro método anticonceptivo seguro y eficaz (como un método de barrera, p. ej., preservativos) mientras utilice Inovelon.
Informe a su médico si utiliza adelgazantes de la sangre, como warfarina. Puede ser que el médico tenga que ajustarle la dosis.
Informe a su médico si utiliza digoxina (un medicamento que se utiliza para tratar enfermedades cardiacas). Puede ser que el médico tenga que ajustarle la dosis.
Toma de Inovelon con alimentos y bebidas
Ver sección 3 “Cómo usar Inovelon” para las recomendaciones sobre la toma de Inovelon con alimentos y bebidas.
Embarazo, lactancia y fertilidad
Si está embarazada o cree que podría estarlo, o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. Solo debe tomar Inovelon durante el embarazo si el médico así lo indica.
Se le aconseja no dar el pecho mientras tome Inovelon ya que se desconoce si rufinamida pasa a la leche materna.
Si usted es mujer en edad fértil, debe utilizar métodos anticonceptivos mientras tome Inovelon.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento al mismo tiempo que Inovelon.
Conducción y uso de máquinas
Inovelon puede hacer que se sienta mareado, somnoliento y afectarle a la visión, especialmente al comienzo del tratamiento o después de un aumento de la dosis. Si le sucede esto, no conduzca ni utilice máquinas.
Inovelon contiene sorbitol (E420)
Inovelon contiene 175 mg de sorbitol (E420) en cada miligramo. El sorbitol es una fuente de fructosa. Si su médico le ha indicado que usted (o su hijo) padecen una intolerancia a ciertos azúcares, o se les ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte usted (o su hijo) con su médico antes de tomar este medicamento.
El sorbitol puede provocar malestar gastrointestinal y un ligero efecto laxante.
Tomar Inovelon con otro medicamento antiepiléptico, que contenga sorbitol, puede afectar a su funcionamiento. Informe a su médico o farmacéutico si está tomando medicamentos antiepilépticos con sorbitol.
Inovelon contiene ácido benzoico (E210)
Inovelon contiene menos de 0,01 mg de ácido benzoico (E210) en cada miligramo. El ácido benzoico puede aumentar el riesgo de ictericia (coloración amarillenta de la piel y los ojos) en los recién nacidos hasta de 4 semanas de edad.
Inovelon contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis diaria; esto es, esencialmente “exento de sodio”.
Inovelon contiene parahidroxibenzoato de metilo (E218) y parahidroxibenzoato de propilo
Estos componentes pueden causar reacciones alérgicas (posiblemente tardías).
3. Cómo usar Inovelon
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Encontrar la mejor dosis de Inovelon para usted puede llevar un tiempo. El médico calculará la dosis adecuada teniendo en cuenta su edad, peso y de si está tomando Inovelon junto con otro medicamento llamado valproato.
Niños de entre uno y cuatro años de edad
La dosis de inicio recomendada es de 10 mg (0,25 ml) por cada kilogramo de peso corporal al día. Se toman dos dosis iguales, la mitad por la mañana y la otra mitad por la noche. El médico calculará su dosis y la podrá aumentar en incrementos de 10 mg (0,25 ml) por cada kilogramo de peso corporal cada tres días.
La dosis diaria máxima dependerá de si está o no tomando valproato también. La dosis diaria máxima sin tomar valproato es de 45 mg (1,125 ml) por cada kilogramo de peso corporal al día. La dosis diaria máxima tomando valproato es de 30 mg (0,75 ml) por cada kilogramo de peso corporal al día.
Niños de 4 años o más que pesan menos de 30 kg
La dosis de inicio recomendada es de 200 mg (5 ml) al día. Se toman dos dosis iguales, la mitad por la mañana y la otra mitad por la noche.
El médico calculará su dosis y la podrá aumentar en 200 mg (5 ml) cada tres días.
La dosis diaria máxima dependerá de si está o no tomando valproato. La dosis diaria máxima sin tomar valproato es de 1.000 mg (25 ml) al día. La dosis diaria máxima tomando valproato es de 600 mg (15 ml) al día.
Adultos, adolescentes y niños que pesan 30 kg o más
La dosis de inicio recomendada es de 400 mg (10 ml) al día. Se toman dos dosis iguales, la mitad por la mañana y la otra mitad por la noche.
El médico calculará su dosis y la podrá aumentar en 400 mg (10 ml) en días alternos.
La dosis diaria máxima dependerá de si está o no tomando valproato. La dosis diaria máxima sin tomar valproato no puede superar los 3.200 mg (80 ml), dependiendo de su peso corporal. La dosis diaria máxima tomando valproato no puede superar los 2.200 mg (55 ml), dependiendo de su peso corporal.
Algunos pacientes pueden responder a dosis menores y el médico podrá ajustarle la dosis en función de su respuesta al tratamiento.
Si presenta efectos adversos, el médico podrá aumentarle la dosis de forma más lenta.
Inovelon suspensión oral se debe tomar dos veces al día, una por la mañana y otra por la noche. Inovelon se debe tomar con alimentos.
Forma de administración
Utilice la jeringa y el adaptador suministrados para la administración.
A continuación se facilitan las instrucciones de uso de la jeringa y del adaptador:
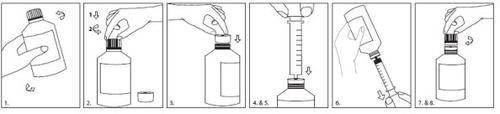
- Agite bien antes del uso.
- Presione y gire el tapón para abrir el frasco.
- Introduzca el adaptador en el cuello del frasco hasta que quede herméticamente cerrado.
- Meta totalmente el émbolo de la jeringa.
- Introduzca la jeringa en la apertura del adaptador el máximo posible.
- Ponga el frasco boca abajo y extraiga la cantidad prescrita de Inovelon.
- Ponga el frasco boca arriba y saque la jeringa.
- Deje el adaptador colocado y vuelva a poner el tapón al frasco. Lave la jeringa con agua limpia y séquela bien.
No reduzca la dosis ni deje de tomar este medicamento a menos que se lo indique su médico.
Si toma más Inovelon del que debe
Si puede haber tomado más Inovelon del que debe, informe al médico o farmacéutico inmediatamente, o póngase en contacto con el servicio de urgencias del hospital más cercano, y lleve el medicamento.
Si olvidó tomar Inovelon
Si olvidó tomar una dosis, continúe tomando el medicamento de la forma habitual. No tome una dosis doble para compensar la dosis olvidada. Si olvida tomar más de una dosis, consulte al médico.
Si interrumpe el tratamiento con Inovelon
Si el médico le indica que deje el tratamiento, siga sus instrucciones respecto a la reducción paulatina de Inovelon para reducir el riesgo de un aumento de las crisis convulsivas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Inovelon puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes efectos adversos pueden ser muy graves:
Erupción cutánea y/o fiebre. Pueden ser signos de una reacción alérgica. Si le ocurre, informe a su médico o acuda al hospital inmediatamente.
Cambio en los tipos de crisis que presenta/crisis más frecuentes que duran un tiempo largo (llamadas estado epiléptico). Informe a su médico inmediatamente.
Un pequeño número de personas en tratamiento con antiepilépticos como Inovelon han tenido pensamientos autolesivos o suicidas. Si en algún momento tiene estos pensamientos, póngase en contacto con su médico inmediatamente (ver sección 2).
Puede presentar los siguientes efectos adversos con este medicamento. Informe al médico si presenta cualquiera de los siguientes efectos:
Los efectos adversos muy frecuentes (más de 1 de cada 10 pacientes) de Inovelon son:
Mareos, dolor de cabeza, náuseas, vómitos, somnolencia, fatiga.
Los efectos adversos frecuentes (más de 1 de cada 100 pacientes) de Inovelon son:
Problemas asociados con el sistema nervioso que incluyen: dificultad para caminar, movimientos anómalos, convulsiones/crisis convulsivas, movimientos inusuales del ojo, visión borrosa, temblores.
Problemas asociados con el estómago que incluyen: dolor de estómago, estreñimiento, indigestión, heces blandas (diarrea), pérdida o cambios en el apetito, pérdida de peso.
Infecciones: infección de oído, gripe, congestión nasal, infección pulmonar.
Además, los pacientes han presentado: ansiedad, insomnio, hemorragia nasal, acné, erupción cutánea, dolor de espalda, menstruaciones infrecuentes, moratones, lesiones craneoencefálicas (como consecuencia de una lesión accidental durante una crisis epiléptica).
Los efectos adversos poco frecuentes (entre 1 de cada 100 y 1 de cada 1.000 pacientes) de Inovelon son:
Reacciones alérgicas y un aumento de los marcadores de la función hepática (aumento de las enzimas hepáticas).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También comunicarlos directamente a través del Sistema Español de Farmacovigilancia demedicamentos de Uso Humano www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Inovelon
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del frasco y en la caja. La fecha de caducidad es el último día del mes que se indica.
Si transcurren más de 90 días después de la primera apertura, la suspensión que quede en el frasco no se debe utilizar.
No utilice la suspensión si observa que el aspecto o el olor del medicamento han cambiado. Lleve el medicamento a su farmacéutico.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Inovelon
- El principio activo es rufinamida. Cada mililitro contiene 40 mg de rufinamida. 5 ml contienen 200 mg de rufinamida.
- Los demás componentes son celulosa microcristalina y carmelosa sódica, ácido cítrico anhidro, simeticona emulsión 30 % (que contiene agua purificada, aceite de silicona, polisorbato 65, metilcelulosa, silica gel, estearato de polietilenglicol, ácido sórbico, ácido benzoico (E210) y ácido sulfúrico), poloxámero 188, aroma de naranja, hidroxietilcelulosa, parahidroxibenzoato de metilo (E218), sorbato potásico (E202), parahidroxibenzoato de propilo, propilenglicol (E1520), sorbitol (E420), líquido (no cristalizante) y agua purificada.
Aspecto del producto y contenido del envase
- Inovelon es una suspensión ligeramente viscosa de color blanco. Viene en un frasco de 460 ml con dos jeringas idénticas y un adaptador para el frasco a presión (PIBA). Las jeringas están graduadas en incrementos de 0,5 ml.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Eisai GmbH
Edmund-Rumpler-Straße 3
60549 Frankfurt am Main
Alemania
e-mail: [email protected]
Responsable de la fabricación:
.
Eisai GmbH
Edmund-Rumpler-Straße 3
60549 Frankfurt am Main
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Eisai SA/NV Tél/Tel: +32 (0)800 158 58 | Lietuva Eisai GmbH Tel: + 49 (0) 69 66 58 50 |
???????? Eisai GmbH Te?.: + 49 (0) 69 66 58 50 | Luxembourg/Luxemburg Eisai SA/NV Tél/Tel: +32 (0)800 158 58 (Belgique/Belgien) |
Ceská republika Eisai GesmbH organizacni složka Tel: + 420 242 485 839 | Magyarország Eisai GmbH Tel.: + 49 (0) 69 66 58 50 |
Danmark Eisai AB Tlf: + 46 (0) 8 501 01 600 (Sverige) | Malta Associated Drug Co. Ltd Tel: + 356 2277 8000 |
Deutschland Eisa GmbH Tel: + 49 (0) 69 66 58 50 | Nederland Eisai B.V. Tél/Tel: + 31 (0) 900 575 3340 |
Eesti Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Saksamaa) | Norge Eisai AB Tlf: + 46 (0) 8 501 01 600 (Sverige) |
Ελλ?δα Arriani Pharmaceutical S.A. Τηλ: + 30 210 668 3000 | Österreich Eisai GesmbH Tel: + 43 (0) 1 535 1980-0 |
España Eisai Farmacéutica, S.A. Tel: + (34) 91 455 94 55 | Polska Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Niemcy) |
France Eisai SAS Tél: + (33) 1 47 67 00 05 | Portugal Eisai Farmacêtica, Unipessoal Lda Tel: + 351 214 875 540 |
Hrvatska Eisai GmbH Tel: + 49 (0) 69 66 58 50 | România Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germania) |
Ireland Eisai GmbH Tel: + 49 (0) 69 66 58 50 | Slovenija Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Nemcija) |
Ísland Eisai AB Sími: + 46 (0)8 501 01 600 (Svíþjóð) | Slovenská republika Eisai GesmbH organizacni složka Tel.: + 420 242 485 839 (Ceská republika) |
Italia Eisai S.r.l. Tel: + 39 02 5181401 | Suomi/Finland Eisai AB Puh/Tel: + 46 (0) 8 501 01 600 (Ruotsi) |
Κ?προς Arriani Pharmaceuticals S.A. Τηλ: + 30 210 668 3000 (Ελλ?δα) | Sverige Eisai AB Tel: + 46 (0) 8 501 01 600 |
Latvija Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Vacija) | United Kingdom (Northern Ireland) Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a INOVELON 40 mg/ml SUSPENSION ORALForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: RufinamidaFabricante: Eisai GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 200 mgPrincipio activo: RufinamidaFabricante: Eisai GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 400 mgPrincipio activo: RufinamidaFabricante: Eisai GmbhRequiere receta
Médicos online para INOVELON 40 mg/ml SUSPENSION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de INOVELON 40 mg/ml SUSPENSION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








