
IBIS 6 MG/ML COLIRIO EN SOLUCION

Cómo usar IBIS 6 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ibis 6 mg/ml colirio en solución
bilastina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ibis y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ibis
- Cómo usar Ibis
- Posibles efectos adversos
- Conservación de Ibis
- Contenido del envase e información adicional
1. Qué es Ibis y para qué se utiliza
Este medicamento contiene bilastina que pertenece a un grupo de medicamentos llamados antihistamínicos. Los antihistamínicos funcionan previniendo los efectos de una sustancia llamada histamina que el cuerpo produce como parte de una reacción alérgica.
Este medicamento se usa para tratar los signos y síntomas de trastornos oculares que se presentan con la conjuntivitis alérgica estacionalen adultos y niños a partir de 2 años.
Este medicamento también se usa para tratar los signos y síntomas de trastornos oculares causados por una alergia a sustancias como los ácaros del polvo doméstico o el pelo de los animales (conjuntivitis alérgica perenne)en adultos y niños a partir de 2 años.
2. Qué necesita saber antes de empezar a usar Ibis
No use Ibis
- si es alérgico a bilastina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Ibis si durante el tratamiento se producen efectos adversos, como irritación ocular, dolor, enrojecimiento o alteraciones en la visión o si su enfermedad empeora. Puede ser necesario interrumpir el tratamiento.
Después de administrar el colirio antialérgico de Ibis en el saco conjuntival del ojo, la agudeza visual puede disminuir durante unos minutos debido a la formación de manchas.
En el caso de inflamación, incluida la conjuntivitis alérgica, consulte a su oftalmólogo si puede utilizar lentes de contacto a pesar de los síntomas.
Niños y adolescentes
Este medicamento está indicado en adultos y niños a partir de 2 años.
No administre este medicamento a niños menores de 2 años debido a que no se ha estudiado la eficacia y seguridad en estos grupos.
Otros medicamentos e Ibis
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Si está usando otros medicamentos para uso ocular, deje al menos 5 minutos entre cada medicamento.
Las pomadas oftálmicas deben administrarse en último lugar.
Embarazo, lactancia y fertilidad
Ibis se puede utilizar durante el embarazo y la lactancia. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Después de la instilación de este medicamento se puede producir visión borrosa temporal u otras alteraciones visuales que afectan a la capacidad de conducir o usar máquinas. Espere hasta que la visión sea nítida antes de conducir o usar maquinaria.
Lentes de contacto
El uso de este medicamento no afecta a las propiedades de las lentes de contacto. Puede seguir usando lentes de contacto durante el uso de este medicamento.
Debe quitarse las lentes de contacto antes de la aplicación del colirio y no se las vuelva a poner hasta 15 minutos después de la administración.
3. Cómo usar Ibis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada para adultos y niños a partir de 2 años es de una gota en cada ojo afectado una vez al día.
Este medicamento se puede usar hasta un máximo de 8 semanas. Su médico decidirá y le aconsejará sobre cuánto tiempo debe usarlo en función de su situación.
Solo para uso ocular.
Forma de administración
- Lávese siempre las manos y séquelas con una toalla limpia antes de administrar este medicamento.
- Limpie suavemente los párpados si hubiera secreción frotando el párpado con el ojo cerrado desde la parte interna hasta la parte externa con un algodón humedecido con agua tibia.
- Abra el frasco y evite el contacto de la punta del gotero con su ojo o cualquier otra cosa: las gotas y los goteros deben mantenerse limpios.
- Incline la cabeza hacia atrás, o acuéstese, y mire hacia arriba (Figura 1). Usando su dedo, tire del párpado inferior hacia abajo (Figura 2).
- Mire hacia arriba y apriete para que caiga una gota en el ojo.
- Suelte el párpado inferior y mantenga el ojo cerrado un rato para repartir la gota por la superficie del ojo (Figura 3).
- Repita la acción anterior en el otro ojo si es necesario.
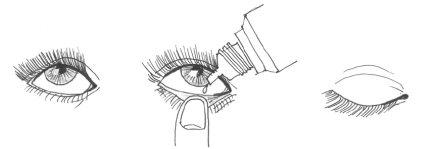

Para evitar la contaminación durante el uso de este medicamento, no toque ninguna superficie (párpados, áreas alrededor del ojo u otras superficies) con la punta del gotero y seque la punta del gotero después de su uso con un pañuelo de papel limpio para eliminar cualquier líquido residual.
Si usa más Ibis del que debe
Puede enjuagarlo con agua tibia. En caso de duda, consulte con su médico. Asimismo, en caso de sobredosis o ingestión accidental, puede consultar al Servicio de Información Toxicológica Tel.: 91 562 04 20.
Si olvidó usar Ibis
No use una dosis doble para compensar las dosis olvidadas.
Si olvida aplicar la gota a tiempo, aplique la gota olvidada lo antes posible y luego vuelva a su pauta de dosificación habitual.
Si interrumpe el tratamiento con Ibis
El tratamiento con este medicamento debe realizarse si es posible de forma regular hasta que se alivien los síntomas. Si deja de usar Ibis mientras aún está expuesto a los alérgenos cabe esperar que vuelvan a aparecer los síntomas típicos de la alergia.
Si tiene cualquier otra duda sobre el uso de este medicamento, consulte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado los siguientes efectos adversos.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
Alteración del gusto (disgeusia), dolor de cabeza.
Ojo seco, secreción ocular, irritación ocular, aumento de la producción de lágrimas, molestias oculares.
En caso de que se produzca uno de los efectos adversos descritos anteriormente, deje de usar este medicamento y consulte directamente a su médico. Los efectos adversos mencionados suelen ser leves y desaparecen rápidamente en todos los casos. Por lo tanto, no se requieren medidas específicas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ibis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta o en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Después de la primera apertura del frasco:no use este medicamento si el frasco ha estado abierto más de 2 meses.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ibis
- El principio activo es bilastina 6 mg/ml.
Una gota contiene 0,2 mg de bilastina.
- Los demás componentes son hidroxipropil betadex, metilcelulosa, hialuronato de sodio, glicerol (E 422), hidróxido de sodio 1 N (para el ajuste de pH), agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ibis son gotas oftálmicas transparentes e incoloras, contenidas en 1 frasco multidosis de LDPE blanco que contiene 5 ml de solución libre de conservantes, con gotero de HDPE blanco y sistema precinto de seguridad antimanipulación.
Tamaño de envase: 1 frasco de 5 ml.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Menarini International Operations Luxembourg, S.A.
1, Avenue de la Gare
L-1611 Luxemburgo
Representante local:
Laboratorios Menarini, S.A.
Alfons XII, 587 – 08918 Badalona (Barcelona), España
Responsable de la fabricación
FAMAR Health Care Services Madrid, S.A.U.
Avenida Leganés, 62
28923 Alcorcón, Madrid
España
Este medicamento está autorizado en los Estados Miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania: Bilaxten 6 mg/ml Augentropfen, Lösung;
Austria: Olisir 6 mg/ml Augentropfen, Lösung;
Bélgica: Bellozal 6 mg/ml gotas oftálmicas, solución;
Croacia: Nixar 6 mg/ml kapi za oko, otopina;
Chipre: Bilaz 6mg/ml οφθαλμικ?ς σταγ?νες;
Eslovenia: Bilador 6 mg/ml kapljice za oko, raztopina;
España: Ibis 6 mg/ml colirio en solución;
Estonia: Opexa;
Francia: Bilaska 6 mg/ml collyre en solución;
Grecia: Bilaz;
Hungría: Lendin 6 mg/ml szemcsepp;
Irlanda: Drynol 6 mg/ml gotas oftálmicas, solución;
Italia: Olisir 6 mg/ml collirio, soluzione;
Letonia: Opexa 6 mg/ml acu pilieni, škidums;
Lituania: Opexa 6 mg/ml akiu lašai, tirpalas;
Luxemburgo: Bellozal 6 mg/ml gotas oftálmicas, solución;
Malta: Gosall 6 mg/ml gotas oftálmicas, solución;
Polonia: Clatra;
Portugal: Lergonix 6 mg/ml colírio, solução;
República Checa: Xados;
República Eslovaca: Omarit 6 mg/ml ocná instilácia;
Rumanía: Borenar 6 mg/ml picaturi oftalmice, solutie.
Fecha de la última revisión de esteprospecto:Junio 2025
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Precio medio en farmacia18.8 EUR
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IBIS 6 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 6 mg/mlPrincipio activo: BilastinaFabricante: Faes Farma S.A.Requiere recetaForma farmacéutica: COLIRIO, 0,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: COLIRIO, 1 mg/mlPrincipio activo: olopatadinaFabricante: Tiedra Farmaceutica S.L.Requiere receta
Médicos online para IBIS 6 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IBIS 6 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















