
IBEROGAST GOTAS ORALES EN SOLUCION


Cómo usar IBEROGAST GOTAS ORALES EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO : INFORMACIÓN PARA EL PACIENTE
Iberogast
Gotas orales en solución
Extractos líquidos etanólicos de carraspique blanco, raíces de angélica, flores de manzanilla, frutos de alcaravea, frutos de cardo mariano, hojas de melisa, hojas de menta, celidonia, raíz de regaliz.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora, o si no mejora después de 7 días de tratamiento.
Contenido del prospecto
- Qué es Iberogast y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Iberogast
- Cómo tomar Iberogast
- Posibles efectos adversos
- Conservación de Iberogast
- Contenido del envase e información adicional
1. Qué es Iberogast y para qué se utiliza
Iberogast es un medicamento a base de plantas que contiene extractos líquidos etanólicos de carraspique blanco, raíces de angélica, flores de manzanilla, frutos de alcaravea, frutos de cardo mariano, hojas de melisa, hojas de menta, celidonia, raíz de regaliz.
Este medicamento está indicado para el tratamiento de trastornos gastrointestinales tales como dispepsia (trastorno de la digestión) y gastritis (inflamación del estómago), así como en el alivio de los síntomas asociados, dolor de estómago, hinchazón abdominal, flatulencia, cólicos gastrointestinales, náuseas y ardor de estómago.
Iberogast está indicado en adultos y adolescentes mayores de 12 años.
Debe consultar a un médico si empeora, o si no mejora después de 7 días de tratamiento.
2. Qué necesita saber antes de empezar a tomar Iberogast
No tome Iberogast
Si es alérgico a los principios activos o a cualquier otro componente de este medicamento (Incluidos en la sección 6).
Si padece o ha padecido una enfermedad del hígado o si está tomando medicamentos que incluyan daño hepático como efecto secundario en el prospecto. En caso de duda, consulte a su médico o farmacéutico.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero si los síntomas persisten o si no se obtienen los resultados esperados a pesar del tratamiento.
Esté atento ante los signos y síntomas que puedan indicarle que su hígado no está funcionando adecuadamente.
Si nota que la piel o los ojos están amarillentos, la orina es oscura, las heces están descoloridas o hay dolor en la parte superior del abdomen, interrumpa inmediatamente el tratamiento con Iberogast y consulte con su médico. Estos pueden ser síntomas de daño hepático.
Niños y Adolescentes
No deben utilizar este medicamento los niños menores de 12 años, pues no se dispone de información clínica suficiente.
Si sus síntomas no mejoran en 7 días o incluso empeoran, consulte con su médico para que pueda excluir otras enfermedades graves.
Otros medicamentos e Iberogast
No se conocen interacciones con otros medicamentos hasta la fecha.
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente otros medicamentos, o podría tener que tomar cualquier otro medicamento.
Toma de Iberogast junto con alimentos y bebidas
No se conocen interacciones con alimentos y bebidas.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se han realizado estudios para establecer la seguridad de uso de este medicamento durante el embarazo o la lactancia, por lo que no se debe administrar en estas circunstancias.
Conducción y uso de máquinas
Iberogast contiene etanol (alcohol). No parece que a las dosis recomendadas del medicamento, las cantidades ingeridas de alcohol vayan a alterar la capacidad de conducir ni para manipular máquinas. No obstante, podría disminuir la capacidad de reacción, por lo que no se aconseja conducir vehículos ni manejar maquinaria cuya utilización requiera especial atención, hasta que se compruebe que la capacidad para realizar estas tareas no queda afectada.
Información importante sobre determinados ingredientes de Iberogast
Iberogast contiene un 31 % (V/V) de etanol (alcohol), cantidad que se corresponde con
240 mg de etanol en 20 gotas (por unidad de dosis), lo que equivale a 6,2 ml de cerveza o 2,6 ml de vino. Este medicamento es perjudicial para personas que padecen alcoholismo. El contenido en alcohol debe tenerse en cuenta en el caso de mujeres embarazadas o en periodo de lactancia, niños y poblaciones de alto riesgo, como pacientes con enfermedades hepáticas o epilepsia.
Iberogast contiene menos de 0,1 UC por cada 20 gotas (unidad de dosis).
3. Cómo tomar Iberogast
Siga exactamente las instrucciones de administración de este medicamento contenidas en este prospecto o las indicadas por su médico, farmacéutico o enfermero. En caso de duda, pregunte a su médico, farmacéutico o enfermero.
La dosis normal recomendada es:
Adultos y adolescentes mayores de 12 años: tomar 20 gotas de Iberogast, 3 veces al día antes o durante las comidas, junto con un poco de líquido. El frasco deber inclinarse durante el goteo (en ángulo de 45º).
Si los síntomas persisten, o se agravan tras 7 días de tratamiento, se debe consultar al médico.
Después de 2 meses de uso del medicamento, consulte a su médico sobre la posibilidad de continuar con el tratamiento. La duración de éste, depende del tipo, severidad y evolución de la enfermedad.
Recuerde agitar el medicamento antes de usarlo.
Uso en niños y adolescentes:
No se recomienda la administración de este medicamento a niños menores de 12 años.
Si toma más Iberogast del que debe
Hasta el momento, no ha habido casos de sobredosis aguda. Sin embargo se debe considerar el contenido de alcohol presente en el producto.
En caso de sobredosis, podrían potenciarse los efectos adversos descritos. En caso de ingestión accidental, consulte inmediatamente a su farmacéutico o médico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el producto y la cantidad ingerida.
Si olvido tomar Iberogast
Si ha olvidado la toma de Iberogast, la próxima toma se realizará según lo descrito en las instrucciones de este prospecto o de acuerdo con la dosis que su médico le haya indicado. No tome una dosis doble para compensar la dosis olvidada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Iberogast puede producir efectos adversos, aunque no todas las personas los sufran.
Muy raras(pueden afectar hasta 1 de cada 10.000 personas): reacciones de hipersensibilidad como erupciones cutáneas, picazón o dificultad respiratoria.
Frecuencia no conocida(no puede estimarse la frecuencia a partir de los datos disponibles): se han notificado daños en el hígado (aumento de los valores hepáticos, ictericia relacionada con el medicamento, hepatitis e insuficiencia hepática); si nota síntomas como coloración amarillenta de la piel o los ojos, orina oscura, heces decoloradas, debe interrumpir inmediatamente el tratamiento con Iberogast y consultar a su médico.
En el caso de presentar una reacción al medicamento se debe suspender la administración del medicamento y se debe acudir de inmediato a un médico. Éste podrá evaluar la gravedad de la reacción y determinar cualquier medida adicional necesaria.
Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos no descritos en este prospecto.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Iberogast
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25 ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Consumir Iberogast en las 8 semanas posteriores a la primera apertura del envase.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Iberogast
Cada ml de gotas orales (equivalente a 20 gotas) contiene:
Extracto líquido etanólico (50% (V/V)):
0,15 ml de plantas frescas de Iberis amara L, carraspique blanco (1:1,5-2,5);
Extractos líquidos etanólicos (30 % (V/V)):
0,2 ml de flores de Matricaria recutita L, manzanilla (1: 2-4);
0,1 ml de raíz de Angelica archangelica L, angélica (1: 2,5-3,5);
0,1 ml de fruto de Carum carvi L, alcaravea (1: 2,5-3,5);
0,1 ml de Chelidonium majus L, celidonia (1: 2,5-3,5);
0,1 ml de raíz de Glycyrrhiza glabra L, regaliz (1: 2,5-3,5);
0,1 ml de hoja de Melissa officinalis L, melisa (1: 2,5-3,5);
0,1 ml de fruto de Silybum marianum L Gaertner, cardo mariano (1: 2,5-3,5) y
0,05 ml de hoja de Mentha piperita L, menta (1: 2,5-3,5)
Los demás componentes (excipiente(s)) son:
El medicamento contiene un 31 % (V/V) de etanol.
Aspecto del producto y contenido del envase
Líquido marrón oscuro, transparente o ligeramente turbio con un olor característico y sabor amargo.
Por las características del medicamento podría ocurrir que Iberogast presentara precipitados o turbidez, si esto ocurriera, no tendría efecto en cuanto a la eficacia del preparado.
El medicamento se presenta en frasco de vidrio topacio con cuentagotas y cierre con tapón de rosca. Se presenta en 3 tamaños de envase: 20 ml, 50 ml y 100 ml
Titular de la autorización de comercialización
Bayer Hispania, S.L.
Av. Baix Llobregat, 3-5
08970 Sant Joan Despí (Barcelona)
Responsable de la fabricación:
Steigerwald Arzneimittelwerk GmbH
Havelstrasse 5
Darmstadt D-64295
Alemania
Fecha de la última revisión de este prospecto: Abril 2019
“La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.es/”
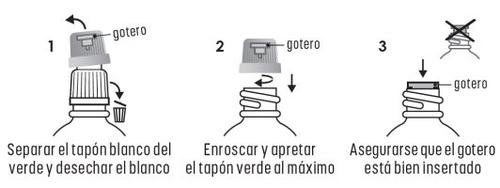
INSTRUCCIONES PARA EL USO DEL GOTERO
- País de registro
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IBEROGAST GOTAS ORALES EN SOLUCIONForma farmacéutica: COMPRIMIDO MASTICABLE, 40 mg simeticonaPrincipio activo: siliconesFabricante: Uriach Consumer Healthcare S.L.No requiere recetaForma farmacéutica: COMPRIMIDO MASTICABLE, 120 mgPrincipio activo: siliconesFabricante: Uriach Consumer Healthcare S.L.No requiere recetaForma farmacéutica: CAPSULA, 240 MGPrincipio activo: siliconesFabricante: Uriach Consumer Healthcare S.L.No requiere receta
Médicos online para IBEROGAST GOTAS ORALES EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IBEROGAST GOTAS ORALES EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















