HEXYON SUSPENSION INYECTABLE EN JERINGA PRECARGADA


Cómo usar HEXYON SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Hexyon suspensión inyectable en jeringa precargada
Vacuna de difteria, tétanos, tos ferina (componente acelular), hepatitis B (rADN), poliomielitis (inactivada), y Haemophilus influenzaede tipo b conjugada (adsorbida)
Lea todo el prospecto detenidamente antes de que su hijo sea vacunado porque contiene información importante.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si su hijo experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Hexyon y para qué se utiliza
- Qué necesita saber antes de que se le administre Hexyon a su hijo
- Cómo usar Hexyon
- Posibles efectos adversos
- Conservación de Hexyon
- Contenido del envase e información adicional
1. Qué es Hexyon y para qué se utiliza
Hexyon (DtaP-IPV-HB-Hib) es una vacuna utilizada para proteger frente a enfermedades infecciosas.
Hexyon ayuda a proteger frente a la difteria, el tétanos, la tos ferina, la hepatitis B, la poliomielitis y las enfermedades graves causadas por el Haemophilus influenzaede tipo b. Hexyon se administra a niños a partir de las seis semanas de edad.
La vacuna actúa haciendo que el cuerpo genere su propia protección (anticuerpos) frente a las bacterias y los virus que ocasionan estas diferentes infecciones:
- La difteria es una enfermedad infecciosa que suele afectar primero a la garganta. En la garganta la infección provoca dolor e hinchazón que puede llegar a provocar asfixia. La bacteria causante de esta enfermedad también produce una toxina (veneno) que puede dañar el corazón, los riñones y los nervios.
- El tétanos suele producirse por la penetración de la bacteria del tétanos en una herida profunda. La bacteria produce una toxina (veneno) que provoca espasmos de los músculos, dando lugar a una incapacidad para respirar y la posibilidad de asfixia.
- La tos ferina (llamada frecuentemente pertussis) es una enfermedad altamente contagiosa que afecta a las vías respiratorias. Esto provoca ataques de tos graves que pueden ocasionar problemas respiratorios. Los ataques de tos presentan frecuentemente un "ruido inspiratorio". La tos puede durar de uno a dos meses o más. La tos ferina también puede causar infecciones de oído, infecciones de pecho (bronquitis), que pueden durar un largo tiempo, infecciones pulmonares (neumonía), convulsiones, daño cerebral e incluso la muerte.
- La hepatitis B es causada por el virus de la hepatitis B. Esto provoca que el hígado se hinche (inflamación). En algunas personas el virus puede permanecer en el organismo durante un largo tiempo, y finalmente puede provocar problemas graves en el hígado, incluyendo cáncer de hígado.
- La poliomielitis (llamada frecuentemente polio) es provocada por virus que afectan los nervios. Puede dar lugar a una parálisis o debilidad muscular más frecuentemente de las piernas. La parálisis de los músculos que controlan la respiración y la deglución puede ser mortal.
- Las infecciones por Haemophilus influenzae tipo b(a menudo denominada simplemente Hib) son infecciones bacterianas graves y pueden provocar meningitis (inflamación de las membranas que envuelven el cerebro), que puede producir daño cerebral, sordera, epilepsia o ceguera parcial. La infección también puede provocar inflamación e hinchazón de la garganta, provocando dificultad para tragar y respirar. La infección puede afectar otras partes del cuerpo tales como la sangre, los pulmones, la piel, los huesos y las articulaciones.
Información importante sobre la protección proporcionada
- Hexyon solo ayudará a prevenir estas enfermedades si están causadas por las bacterias o los virus para los cuales la vacuna está destinada. Su hijo podría contraer enfermedades con síntomas similares provocadas por otras bacterias o virus.
- La vacuna no contiene ninguna bacteria o virus vivo y no puede provocar ninguna de las enfermedades infecciosas frente a las que protege.
- Esta vacuna no protege frente a infecciones causadas por otros tipos de Haemophilus influenzaeo frente a la meningitis provocada por otros microorganismos.
- Hexyon no protegerá frente a hepatitis infecciosas causadas por otros agentes como la hepatitis A, hepatitis C y hepatitis E.
- Debido a que los síntomas de la hepatitis B tardan un tiempo largo en desarrollarse, es posible que una infección no identificada de hepatitis B esté presente en el momento de la vacunación. En dichos casos, es posible que la vacuna no evite la infección por hepatitis B.
- Al igual que con cualquier otra vacuna, es posible que Hexyon no proteja al 100% de los niños vacunados.
2. Qué necesita saber antes de que se le administre Hexyon a su hijo
Con el fin de garantizar que Hexyon es adecuado para su hijo, es importante que informe a su médico o enfermero si su hijo presenta alguna de las características detalladas a continuación. Si hay algo que no entiende, consulte a su médico, farmacéutico o enfermero.
No use Hexyon si su hijo:
- ha tenido un trastorno respiratorio o hinchazón de la cara (reacción anafiláctica) tras la administración de Hexyon.
- ha tenido una reacción alérgica
- a los principios activos,
- a cualquiera de los demás componentes incluidos en la sección 6,
- al glutaraldehído, formaldehído, neomicina, estreptomicina y polimixina B, ya que estas sustancias se utilizan durante el proceso de fabricación,
- tras la administración previa de Hexyon o cualquier otra vacuna que contenga difteria, tétanos, tos ferina, poliomielitis, hepatitis B o Hib.
- ha padecido una reacción grave que afecta al cerebro (encefalopatía) en los 7 días posteriores a la administración de una dosis previa de una vacuna frente a la tos ferina (acelular o de célula entera).
- tiene una enfermedad no controlada o enfermedad grave que afecte al cerebro y al sistema nervioso (trastorno neurológico no controlado) o una epilepsia no controlada.
Advertencias y precauciones
Antes de la vacunación consulte a su médico, farmacéutico o enfermero si su hijo:
- tiene una temperatura moderada o alta o una enfermedad aguda (fiebre, dolor de garganta, tos, resfriado o gripe). Es posible que la vacunación con Hexyon deba retrasarse hasta que su hijo se sienta mejor.
- ha padecido alguno de los siguientes acontecimientos adversos después de recibir una vacuna frente a la tos ferina, deberá evaluarse cuidadosamente la decisión de administrar otras dosis de vacuna que contengan el componente pertussis:
- fiebre de 40 ºC o superior dentro de las 48 horas posteriores a la vacunación que no fue debida a otra causa identificable.
- colapso o estado similar al shock con episodio hipotónico–hiporreactivo (debilidad) en las 48 horas siguientes a la vacunación.
- llanto inconsolable, persistente durante 3 horas o más, producido en las 48 horas siguientes a la vacunación.
- ataques (convulsiones) con o sin fiebre, producidas en los 3 días siguientes a la vacunación.
- ha padecido anteriormente síndrome de Guillain-Barré (inflamación temporal de los nervios que provocan dolor, parálisis y trastornos en la sensibilidad) o neuritis braquial (dolor grave y disminución de la movilidad en brazo y hombro) después de la administración de una vacuna que contenga toxoide tetánico (una forma inactiva del toxoide tetánico). En este caso su médico evaluará la decisión de administrar cualquier vacuna que contenga toxoide tetánico.
- está recibiendo un tratamiento que suprime su sistema inmunológico (las defensas naturales del organismo) o presenta cualquier enfermedad que provoca una inmunodeficiencia. En estos casos, la respuesta inmunológica a la vacuna puede verse disminuida. Por lo tanto, se recomienda retrasar la vacunación hasta el final del tratamiento o de la enfermedad. No obstante, los niños con problemas con su sistema inmunológico durante un largo período de tiempo como la infección por VIH (SIDA) se les puede administrar Hexyon pero la protección podría no ser tan buena como en niños cuyo sistema inmunológico esté sano.
- padece una enfermedad aguda o crónica incluyendo insuficiencia renal crónica o fallo (incapacidad de los riñones para funcionar correctamente).
- padece cualquier enfermedad cerebral no diagnosticada o epilepsia no controlada. Su médico evaluará el beneficio potencial que ofrece la vacunación.
- tiene algún problema de la sangre que provoque la aparición de hematomas con facilidad o sangrado prolongado tras producirse pequeños cortes. Su médico le asesorará sobre la conveniencia o no de administrar Hexyon a su hijo.
Uso de Hexyon con otras vacunas o medicamentos
Informe a su médico o enfermero si su hijo está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento o vacuna.
Hexyon puede administrarse al mismo tiempo que otras vacunas como las vacunas antineumocócica, vacunas frente al sarampión-parotiditis-rubéola, vacunas frente a la varicela, vacunas frente al rotavirus o vacunas antimeningocócicas.
Cuando se administre al mismo tiempo que otras vacunas, Hexyon se le administrará en lugares de inyección diferentes.
Los desmayos pueden ocurrir después, o incluso antes, de cualquier inyección con aguja. Por lo tanto, informe a su médico o enfermero si su hijo se ha desmayado con una inyección anterior.
Hexyon contiene fenilalanina, potasio y sodio
Hexyon contiene 85 microgramos de fenilalanina en cada dosis de 0,5 ml. La fenilalanina puede ser dañina si tiene fenilcetonuria (PKU), un trastorno genético poco común en el que la fenilalanina se acumula porque el cuerpo no puede eliminarla correctamente.
Hexyon contiene menos de 1 mmol de potasio (39 mg) y menos de 1 mmol de sodio (23 mg) por dosis, es decir, esencialmente "exento de potasio" y "exento de sodio".
3. Cómo usar Hexyon
Hexyon se le administrará a su hijo por un médico o enfermero debidamente entrenados en el uso de vacunas y que estén equipados para reaccionar ante cualquier reacción alérgica grave poco frecuente que ocurra tras la inyección (ver sección 4 “Posibles efectos adversos”).
Hexyon se administra mediante inyección en un músculo (vía intramuscular, IM) en la parte superior de la pierna o del brazo de su hijo. La vacuna nunca debe administrarse en un vaso sanguíneo o dentro o debajo de la piel.
La dosis recomendada es la siguiente:
Primer ciclo de vacunación (vacunación primaria)
Su hijo recibirá dos inyecciones administradas en un intervalo de dos meses o tres inyecciones administradas en un intervalo de uno a dos meses (al menos cuatro semanas de intervalo). Esta vacuna debe utilizarse de acuerdo con el calendario de vacunación local.
Inyecciones adicionales (vacunación de recuerdo)
Tras el primer ciclo de vacunación, su hijo recibirá una dosis de recuerdo, de acuerdo con las recomendaciones locales, al menos 6 meses después de la última dosis del primer ciclo de vacunación. Su médico le asesorará sobre cuándo debe administrarse dicha dosis.
Si su hijo no recibe una dosis de Hexyon
Si por olvido, su hijo no recibe una inyección prevista en el calendario, es importante que informe a su médico o enfermero, ellos decidirán cuándo administrar la dosis olvidada.
Es importante seguir las instrucciones del médico o enfermero para que su hijo complete el ciclo de vacunación. De lo contrario, su hijo podría no estar totalmente protegido frente a las enfermedades.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas graves (reacción anafiláctica)
Si cualquiera de estos síntomas ocurre después de abandonar el lugar donde su hijo recibió la inyección, debe consultar a un médico INMEDIATAMENTE:
- dificultad para respirar
- coloración azulada de la lengua o labios
- erupción
- hinchazón de la cara o la garganta
- malestar repentino y grave con una bajada de la presión arterial que causa mareos y pérdida de la conciencia, frecuencia cardíaca acelerada asociada con trastornos respiratorios
Cuando estos signos y síntomas (signos o síntomas de una reacción anafiláctica) se presentan, suelen desarrollarse rápidamente tras la administración de la inyección y mientras el niño todavía está en la clínica o consulta médica.
La posibilidad de que ocurran reacciones alérgicas graves tras recibir esta vacuna es rara (podrían afectar hasta 1 de cada 1.000 personas).
Otros efectos adversos
Si su hijo experimenta cualquiera de los siguientes efectos adversos, consulte a su médico, enfermero o farmacéutico.
- Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas) son:
- pérdida del apetito (anorexia)
- llanto
- adormecimiento (somnolencia)
- vómitos
- fiebre (temperatura de 38 °C o superior)
- irritabilidad
- dolor, enrojecimiento o hinchazón en el lugar de inyección
- Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas) son:
- llanto anormal (llanto prolongado)
- diarrea
- endurecimiento en el lugar de inyección (induración)
- Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas) son:
- reacción alérgica
- fiebre alta (temperatura de 39,6 °C o superior)
- bulto (nódulo) en el lugar de inyección
- Efectos adversos raros (pueden afectar hasta 1 de cada 1.000 personas) son:
- Erupción
- reacciones extensas en el lugar de inyección (mayores de 5 cm), incluyendo extensa hinchazón de un miembro que se extiende desde el lugar de la inyección hasta más allá de una o ambas articulaciones. Estas reacciones comienzan en las 24-72 horas posteriores a la vacunación, pueden estar asociadas con enrojecimiento, calor, dolor a la palpación o dolor en el lugar de la inyección y remiten en el plazo de 3-5 días sin necesidad de tratamiento.
- ataques (convulsiones) con o sin fiebre.
- Efectos adversos muy raros (pueden afectar hasta 1 de cada 10.000 personas) son:
- episodios en los que su hijo entra en un estado similar al shock o esta pálido, debilitado y no responde durante un periodo de tiempo (reacciones hipotónicas o episodios de hipotonía-hiporrespuesta, EHH).
Efectos adversos potenciales
Se han comunicado ocasionalmente otros efectos adversos no mencionados anteriormente con otras vacunas que contienen difteria, tétanos, tos ferina, poliomielitis, hepatitis B o Hib y no directamente con Hexyon:
- Se ha notificado después de la administración de una vacuna que contiene tétanos, inflamación temporal de los nervios que provoca dolor, parálisis y trastornos en la sensibilidad (Síndrome de Guillain-Barré), dolor severo y disminución de la movilidad en el brazo y hombro (neuritis braquial).
- Se han notificado después de la administración de vacunas que contengan el antígeno de hepatitis B, inflamación de los nervios que provocan trastornos sensoriales o debilidad de los brazos y/o piernas (polirradiculoneuritis), parálisis facial, trastornos visuales, oscurecimiento o pérdida repentina de la visión (neuritis óptica), enfermedad inflamatoria del cerebro y la médula espinal (desmielinización del sistema nervioso central, esclerosis múltiple).
- Hinchazón o inflamación del cerebro (encefalopatía/encefalitis).
- En los niños nacidos muy prematuramente (a las 28 semanas de gestación o antes) se pueden producir intervalos entre respiraciones más largos de lo normal durante 2-3 días posteriores a la vacunación.
- Hinchazón de uno o ambos pies y extremidades inferiores. Esto puede producirse junto con una coloración azulada de la piel (cianosis), enrojecimiento, pequeñas áreas de sangrado bajo la piel (púrpura transitoria) y llanto grave, tras la administración de vacunas que contienen Haemophilus influenzaetipo b. Si se produce esta reacción, ocurrirá principalmente después de las primeras inyecciones y se observará en las primeras horas posteriores a la vacunación. Todos los síntomas remitirán completamente en las 24 horas siguientes sin necesidad de tratamiento.
Comunicación de efectos adversos
Si su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Hexyon
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y etiqueta después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C).
No congelar.
Conservar el envase en el embalaje exterior para protegerlo de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo debe deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Hexyon
Los principios activos por dosis (0,5 ml)1 son:
Toxoide diftérico | no menos de 20 UI2,4 (30 Lf) |
Toxoide tetánico | no menos de 40 UI3,4 (10 Lf) |
Antígenos de Bordetella pertussis | |
Toxoide pertussis | 25 microgramos |
Hemaglutinina filamentosa | 25 microgramos |
Poliovirus (inactivado)5 | |
Tipo 1 (Mahoney) | 29 unidades de antígeno D6 |
Tipo 2 (MEF-1) | 7 unidades de antígeno D6 |
Tipo 3 (Saukett) | 26 unidades de antígeno D6 |
Antígeno de superficie del virus de la Hepatitis B7 | 10 microgramos |
Polisacárido de Haemophilus influenzaetipo b | 12 microgramos |
(polirribosilribitol fosfato) | |
conjugado con proteína del tétanos | 22-36 microgramos |
1 Adsorbido en hidróxido de aluminio, hidratado (0,6 mg Al3+)
2 Como límite de confianza inferior (p=0,95) y no menos de 30 UI como valor medio
3 Como límite de confianza inferior (p=0,95)
4 O actividad equivalente determinada por la evaluación de la inmunogenicidad
5 Cultivado en células Vero
6 Estas cantidades de antígeno son estrictamente las mismas que las expresadas anteriormente como 40-8-32 unidades de antígeno D, para virus tipo 1, 2 y 3 respectivamente, cuando se miden por otro método inmunoquímico adecuado.
7 Producido en células de levadura Hansenula polymorpha mediante tecnología de ADN recombinante
Los demás componentes son:
Hidrogenofosfato de disodio, dihidrogenofosfato de potasio, trometamol, sacarosa, aminoácidos esenciales que incluyen L-fenilalanina, hidróxido de sodio y/o ácido acético y/o ácido clorhídrico (para ajuste del pH) y agua para preparaciones inyectables.
La vacuna puede contener trazas de glutaraldehído, formaldehído, neomicina, estreptomicina y polimixina B.
Aspecto del producto y contenido del envase
Hexyon se suministra como suspensión inyectable en jeringa precargada (0,5 ml).
Hexyon está disponible en envases de 1, 10 o 50 jeringas precargadas sin aguja fija.
Hexyon está disponible en envases de 1 o 10 jeringas precargadas con 1 aguja separada.
Hexyon está disponible en envases de 1 o 10 jeringas precargadas con 2 agujas separadas.
Hexyon está disponible en envase múltiple de 5 envases, cada uno de 10 jeringas precargadas sin aguja fija.
Hexyon está disponible en envases de 1 o 10 jeringas precargadas con 1 o 10 agujas de seguridad separadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Después de agitarla, la apariencia normal de la vacuna es una suspensión turbia blanquecina.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Sanofi Winthrop Industrie, 82 Avenue Raspail, 94250 Gentilly, Francia
Responsable de la fabricación
Sanofi Winthrop Industrie, 1541 avenue Marcel Mérieux, 69280 Marcy l'Etoile, Francia
Sanofi Winthrop Industrie, Voie de L’Institut - Parc Industriel d'Incarville, BP 101, 27100 Val de Reuil, Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique /Belgien Sanofi Belgium Tel: +32 2 710.54.00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tel: +32 2 710.54.00 |
Ceská republika Sanofi, s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt Tel: +36 1 505 0055 |
Danmark Sanofi A/S Tel: +45 4516 7000 | Malta Sanofi S.r.l. Tel: +39 02 39394 275 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel: 0800 54 54 010 Tel. aus dem Ausland: +49 69 305 21 130 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge Sanofi-aventis Norge AS Tel: + 47 67 10 71 00 |
Ελλ?δα ΒΙΑΝΕΞ Α.Ε. Τηλ: +30.210.8009111 | Österreich Sanofi-Aventis GmbH Tel: +43 (1) 80185-0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi sp. z o. o. Tel: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tel: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi – Produtos Farmacêuticos, Lda. Tel: + 351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 21 317 31 36 |
Ireland sanofi-aventis Ireland T/A SANOFI Tel: + 353 (0) 1 4035 600 | Slovenija Swixx Biopharma d.o.o Tel: +386 235 51 00 |
Ísland Vistor Tel: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800536389 | Suomi/Finland Sanofi Oy Tel: +358 (0) 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ.: +357 22 741741 | Sverige Sanofi AB Tel: +46 8-634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 6164 750 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
La información más reciente aprobada sobre esta vacuna está disponible en la siguiente URL: https://hexyon.info.sanofi o escaneando el código QR con su teléfono móvil (smartphone):
[Código QR a incluir]
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
- Agitar la jeringa precargada para que el contenido sea homogéneo.
- Hexyon no debe mezclarse con otros medicamentos.
- Hexyon debe administrarse por vía intramuscular. Los lugares de inyección recomendados son el área antero-lateral superior del muslo (lugar preferente) o el músculo deltoides en niños mayores (posiblemente a partir de los 15 meses de edad).
No deben utilizarse las vías intradérmica o intravenosa. No administrar por inyección intravascular: asegúrese de que la aguja no haya penetrado en un vaso sanguíneo.
- No utilice las jeringas precargadas si la caja está dañada.
Preparación para la administración
La jeringa con la suspensión para inyección debe inspeccionarse visualmente antes de la administración. En caso de partículas extrañas, fugas, activación prematura del émbolo o sellado defectuoso de la punta, deseche la jeringa precargada.
La jeringa está diseñada para un solo uso y no debe reutilizarse.
Instrucciones de uso de la jeringa precargada Luer Lock
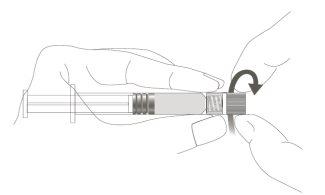
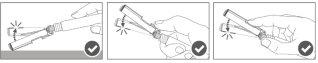
Imagen A: Jeringa Luer Lock con tapón en el extremo rígido
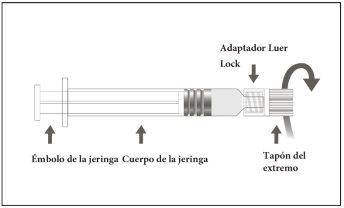
Paso 1:Sujetando el adaptador Luer Lock con una mano (evite sujetar el émbolo o el cuerpo de la jeringa), desenrosque la tapa de la punta girándola. |
|
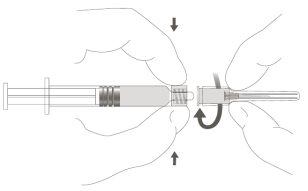
Paso 2:Para conectar la aguja a la jeringa, gire suavemente la aguja en el adaptador Luer Lock de la jeringa hasta que sienta una ligera resistencia. |
|
Instrucciones de uso de la aguja de seguridad con la jeringa Luer Lock
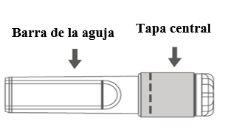
Imagen B: Aguja de seguridad (dentro de la barra) | Imagen C: Componentes de la aguja de seguridad (preparada para su uso) |
|
|
Siga los pasos 1 y 2 descritos anteriormente para preparar la jeringa Luer Lock y fijar la aguja.
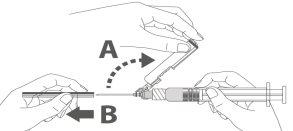
Paso 3:Extraiga el protector de la aguja de seguridad. La aguja está cubierta por el dispositivo de seguridad y el protector. Paso 4: A:Separe el dispositivo de seguridad de la aguja hacia el cuerpo de la jeringa en el ángulo que se muestra. B:Retire el protector en linea recta. |
|
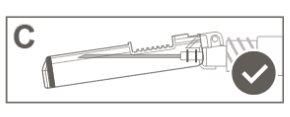
Paso 5:Una vez finalizada la inyección, bloquee (active) el dispositivo de seguridad utilizando una de las tres técnicas ilustradas (3) con una sola mano: activación con una superficie, con el pulgar o con el dedo índice. Nota: La activación se verifica mediante un sonido "clic" y/o táctil. |
|
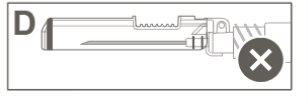
Paso 6:Inspeccionar visualmente la activación del dispositivo de seguridad. El dispositivo de seguridad debe estar completamente bloqueado (activado)como se muestra en la figura C. Nota: Cuando está completamente bloqueado (activado), la aguja debe estar en ángulo con el dispositivo de seguridad. La figura D muestra que el dispositivo de seguridad NO está completamente bloqueado (no activado). |
|
Precaución: No intente desbloquear (desactivar) el dispositivo de seguridad forzando la aguja fuera del dispositivo de seguridad. |
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HEXYON SUSPENSION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 0.5 mlFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 0,5 MLFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para HEXYON SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HEXYON SUSPENSION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes