HEPCLUDEX 2 MG POLVO PARA SOLUCION INYECTABLE


Cómo usar HEPCLUDEX 2 MG POLVO PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Hepcludex 2 mg polvo para solución inyectable
bulevirtida
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Hepcludex y para qué se utiliza
- Qué necesita saber antes de empezar a usar Hepcludex
- Cómo usar Hepcludex
- Posibles efectos adversos
- Conservación de Hepcludex
- Contenido del envase e información adicional
- Guía para la inyección paso a paso
1. Qué es Hepcludex y para qué se utiliza
Qué es Hepcludex
Hepcludex contiene el principio activo bulevirtida, que es un medicamento antiviral.
Para qué se utiliza Hepcludex
Hepcludex se utiliza para tratar la infección a largo plazo (crónica) por el virus de la hepatitis delta (VHD) en adultos con enfermedad hepática compensada (cuando el hígado aún funciona adecuadamente). La infección por el virus de la hepatitis delta causa inflamación del hígado.
Cómo actúa Hepcludex
El VHD utiliza una proteína concreta de las células hepáticas para entrar en dichas células. Bulevirtida, el principio activo de este medicamento, bloquea la proteína y de este modo impide la entrada del VHD en las células hepáticas. Esto reduce la propagación del VHD en el hígado y reduce la inflamación.
2. Qué necesita saber antes de empezar a usar Hepcludex
No tome Hepcludex:
- si es alérgico a bulevirtida o a alguno de los demás componentes de este medicamento (inclui- dos en la sección 6).
Si tiene dudas, consulte a su médico antes de tomar este medicamento.
Advertencias y precauciones
No interrumpa el tratamiento con Hepcludex a menos que su médico le recomiende hacerlo. Interrumpir el tratamiento puede reactivar la infección y empeorar su enfermedad.
Consulte a su médico o farmacéutico antes de empezar a usar Hepcludex:
- si su hígado no funciona adecuadamente – no se sabe en qué medida actúa Hepcludex en esas circunstancias; si su hígado no funciona bien, no se recomienda usar Hepcludex.
- si ha tenido una enfermedad renal o si los análisis indican problemas de riñón. Antes y durante el tratamiento, su médico puede solicitar análisis de sangre para comprobar el correcto funcionamiento de los riñones;
- si padece infección por el VIH o la hepatitis C - no se sabe cómo actúa Hepcludex en estas circunstancias; su médico puede solicitar análisis de sangre para comprobar el estado de la infección por el VIH o la hepatitis C
Niños y adolescentes
Los niños y adolescentes menores de 18 años de edad no deben ser tratados con Hepcludex.
Otros medicamentos y Hepcludex
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Algunos medicamentos pueden aumentar los efectos adversos de Hepcludex y no debe tomarlos al mismo tiempo. Esta es la razón por la que debe informar al médico si está tomando alguno de estos medicamentos:
- ciclosporina, un medicamento que inhibe el sistema inmunitario;
- ezetimiba, utilizado para tratar los niveles elevados de colesterol en sangre;
- irbesartán, utilizado para tratar la hipertensión arterial y las enfermedades cardíacas;
- ritonavir, utilizado para tratar la infección por el VIH;
- sulfasalazina, utilizada para tratar la artritis reumatoide, la colitis ulcerosa y la enfermedad de Crohn.
Algunos medicamentos pueden aumentar o disminuir los efectos de Hepcludex cuando se toman juntos. En algunos casos, puede que se tenga que hacer algunas pruebas o que su médico tenga que modificar la dosis o someterle a controles periódicos:
- tratamientos contra el cáncer (p. ej., dasatinib, docetaxel, ibrutinib o paclitaxel);
- antihistamínicos utilizados para las alergias (p.ej., ebastina o fexofenadina);
- medicamentos para el sistema inmunitario (p.ej., everolimús, sirolimús o tacrolimús);
- medicamentos para el tratamiento de la hepatitis C y el VIH (p.ej., darunavir, glecaprevir, grazoprevir, indinavir, maraviroc, paritaprevir, saquinavir, simeprevir, tipranavir o voxilaprevir);
- medicamentos para la diabetes (p. ej., glibenclamida, nateglinida o repaglinida);
- medicamentos para la disfunción eréctil (p.ej., avanafilo, sildenafilo o vardenafilo);
- medicamentos para el tratamiento de la hipertensión arterial y las enfermedades cardíacas (p.ej., olmesartán, telmisartán o valsartán);
- estatinas, medicamentos usados para los niveles elevados de colesterol en sangre (p.ej. atorvastatina, fluvastatina, lovastatina, pitavastatina, pravastatina, rosuvastatina o simvastatina);
- hormonas tiroideas utilizadas para tratar problemas de tiroides;
- alfentanilo, un medicamento opioide que se utiliza para tratar el dolor intenso;
- bosentán, utilizado para la hipertensión arterial pulmonar;
- buspirona, un medicamento para la ansiedad;
- budesonida, utilizada para el asma y la enfermedad pulmonar obstructiva crónica;
- conivaptán y tolvaptán, utilizados para tratar la hiponatremia (niveles bajos de sodio);
- darifenacina, utilizada para tratar la incontinencia urinaria;
- dronedarona, un medicamento para las arritmias cardíacas;
- eletriptán, utilizado para los dolores de cabeza de tipo migrañoso;
- eplerenona, utilizada para la hipertensión;
- estrona 3-sulfato, un medicamento hormonal para la menopausia;
- felodipino y nisoldipino (medicamentos para el corazón);
- lomitapida, utilizada para los niveles elevados de colesterol en sangre;
- lurasidona y quetiapina, medicamentos antipsicóticos para trastornos psiquiátricos;
- midazolam y triazolam, medicamentos para tratar el insomnio (incapacidad para dormir) y para anestesia (evitar el dolor durante la cirugía);
- naloxegol, utilizado para tratar la dependencia de medicamentos opioides para el dolor intenso;
- ticagrelor, anticoagulante para impedir la coagulación de la sangre.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. No debe usar este medicamento a menos que su médico se lo indique específicamente.
Si es una mujer en edad fértil, no debe usar este medicamento sin utilizar un método anticonceptivo eficaz.
Hable con su médico para decidir si puede dar el pecho durante el tratamiento con Hepcludex. Se desconoce si Hepcludex se excreta en la leche materna. Por consiguiente, se debe decidir interrumpir la lactancia o interrumpir el tratamiento con Hepcludex.
Conducción y uso de máquinas
Mareo y cansancio son efectos adversos que pueden afectar a la capacidad para conducir y utilizar máquinas. Si tiene alguna duda, consulte a su médico.
Hepcludex contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Hepcludex
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Posología
La dosis recomendada es de 2 mg una vez al día en inyección subcutánea (justo debajo de la piel). Su médico le indicará cuánto tiempo debe usar el medicamento.
Su médico y enfermero le enseñarán cómo preparar e inyectar Hepcludex. Este prospecto contiene una guía para la inyección paso a paso para ayudarle a inyectarse el medicamento (ver sección 7).
Si usa más Hepcludex del que debe
La dosis habitual es de 2 mg (1 vial) al día. Si cree que puede haber recibido más de lo debido, dígaselo a su médico inmediatamente.
Si olvidó usar Hepcludex
Si han pasado menos de 4 horas desde que olvidó la dosis de Hepcludex, se debe inyectar esa dosis omitida lo antes posible y administrarse la siguiente dosis programada a la hora habitual.
Si han pasado más de 4 horas desde que olvidó la dosis de Hepcludex, no se debe administrar la dosis omitida. Se debe administrar la próxima dosis el día siguiente a la hora habitual. No se debe administrar una dosis doble para compensar las dosis olvidadas. Informe a su médico si ha olvidado una dosis de Hepcludex.
Si interrumpe el tratamiento con Hepcludex
Si ya no desea seguir usando Hepcludex, consulte a su médico antes de interrumpir el tratamiento. Interrumpir el tratamiento puede reactivar la infección y empeorar su enfermedad. Informe a su médico inmediatamente sobre cualquier cambio en los síntomas tras la interrupción del tratamiento.
Si tiene cualquier otra duda sobre el uso de Hepcludex pregunte a su médico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta cualquier efecto adverso o si observa alguno no mencionado en este prospecto, consulte a su médico.
El siguiente efecto adverso es muy frecuente(puede afectar a más de 1 de cada 10 personas):
- dolor de cabeza.
Los siguientes efectos adversos son frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- mareo
- náuseas
- cansancio
- enfermedad de tipo gripal
- picor
- dolor en las articulaciones
- reacciones en el lugar de la inyección que pueden incluir hinchazón, enrojecimiento, irritación, hematomas, picor, erupción cutánea, induración, infección o dolor local.
Los siguientes efectos adversos son poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
- reacciones alérgicas, incluida una reacción anafiláctica (reacción alérgica potencialmente mortal repentina).
Los síntomas de reacciones alérgicas pueden incluir:
- falta de aliento o sibilancia
- hinchazón de cara, labios, lengua o garganta (angioedema)
- erupciones cutáneas
- cambios en la tensión arterial o la frecuencia cardiaca.
Los síntomas de una reacción anafiláctica son los mismos que los de una reacción alérgica, pero son más intensos y requieren asistencia médica inmediata.
Los análisis de sangre también pueden indicar:
- un aumento de ácidos biliares en sangre (muy frecuentes)
- un aumento de glóbulos blancos (eosinófilos) (frecuentes).
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Hepcludex
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el vial después de «CAD». La fecha de caducidad es el último día del mes que se indica.
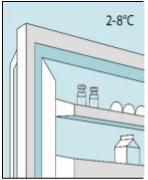
Conservar en la nevera (entre 2 ºC y 8 ºC). Para protegerlos de la luz, mantener los viales en el envase exterior.
La solución reconstituida se debe utilizar inmediatamente. No obstante, si ello no es posible, se puede conservar durante un máximo de 2 horas a una temperatura de hasta 25 ºC.
Los medicamentos o las agujas utilizadas no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de las agujas utilizadas.
6. Contenido del envase e información adicional
Composición de Hepcludex
El principio activo es bulevirtida 2 mg. Cada vial contiene bulevirtida acetato equivalente a 2 mg de bulevirtida.
Los demás componentes son carbonato de sodio anhidro, hidrogenocarbonato de sodio, manitol, ácido clorhídrico e hidróxido sódico.
Aspecto del producto y contenido del envase
Bulevirtida es un polvo para solución inyectable y se presenta en forma de polvo blanco o blanquecino.
Cada caja contiene 30 dosis individuales.
Titular de la autorización de comercialización
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Irlanda
Responsable de la fabricación
LYOCONTRACT GmbH
Pulverwiese 1
38871 Ilsenburg
Alemania
o
Gilead Sciences Ireland UC
IDA Business and Technology Park
Carrigtohill
Co. Cork
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Gilead Sciences Belgium SRL-BV Tél/Tel: + 32 (0) 24 01 35 50 | Lietuva Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
Gilead Sciences Ireland UC ??π.: + 353 (0) 1 686 1888 | Luxembourg/Luxemburg Gilead Sciences Belgium SRL-BV Tél/Tel: + 32 (0) 24 01 35 50 |
Ceská republika Gilead Sciences s.r.o. Tel: + 420 (0) 910 871 986 | Magyarország Gilead Sciences Ireland UC Tel.: + 353 (0) 1 686 1888 |
Danmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Deutschland Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Nederland Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Eesti Gilead Sciences Poland Sp. z o.o. Tel.: +48 (0) 22 262 8702 | Norge Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Ελλáδα Gilead Sciences Ελλáς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Österreich Gilead Sciences GesmbH Tel: + 43 (0) 1 260 830 |
España Gilead Sciences, S.L. Tel: + 34 (0) 91 378 98 30 | Polska Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
France Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 (0) 21 7928790 |
Hrvatska Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | România Gilead Sciences (GSR) S.R.L. Tel: +40 31 631 18 00 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenija Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ísland Gilead Sciences Sweden AB Sími: + 46 (0) 8 5057 1849 | Slovenská republika Gilead Sciences Slovakia s.r.o. Tel: + 421 (0) 232 121 210 |
Italia Gilead Sciences S.r.l. Tel: + 39 02 439201 | Suomi/Finland Gilead Sciences Sweden AB Puh/Tel: + 46 (0) 8 5057 1849 |
Κúπρος Gilead Sciences Ελλáς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Sverige Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvija Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 | United Kingdom (Northern Ireland) Gilead Sciences Ireland UC Tel: + 44 (0) 8000 113 700 |
Fecha de la última revisión de este prospecto: <{MM/AAAA}> <{mes AAAA}>.
Este medicamento se ha autorizado con una «aprobación condicional». Esta modalidad de aprobación significa que se espera obtener más información de este medicamento.
La Agencia Europea de Medicamentos revisará la información nueva de este medicamento al menos una vez al año y este prospecto se actualizará cuando sea necesario.
- Guía para la inyección paso a paso
Antes de usar Hepcludex, debe leer primero las secciones 1-6 de este prospecto.
Antes de iniciar el tratamiento con este medicamento en casa, su médico o enfermero le enseñará cómo preparar e inyectar Hepcludex. En esta guía se indica cómo debe inyectarse el medicamento usted mismo. Consulte a su médico o enfermero si hay algo que no le parezca claro, si tiene alguna pregunta o si necesita más información o ayuda. Tómese el tiempo necesario para preparar e inyectar Hepcludex con cuidado.
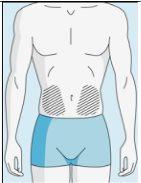
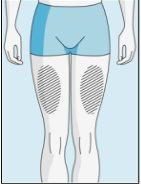
Zonas de inyección | Abdomen | Parte superior del muslo | |
Para reducir las reacciones en el lugar de la inyección, puede cambiar el lugar de inyección de bulevirtida de manera regular. No inyectebulevirtida en las siguientes zonas: rodilla, ingle, parte inferior o interior de las nalgas, directamente en un vaso sanguíneo, alrededor del ombligo, en tejido cicatricial, hematomas, lunares, una cicatriz quirúrgica, tatuajes o quemaduras, o donde se haya producido una reacción en la zona de inyección. |
|
| |
|
|
|
|
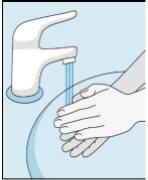
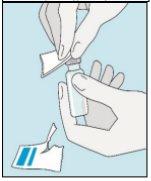
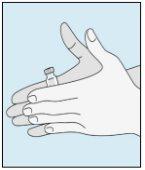
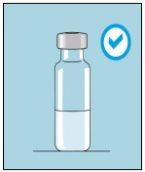
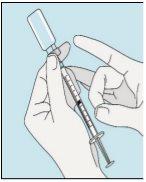
1A Almacenamiento | 1B Mezclar dosis | 1C Lavarse las manos | 1D Limpiar el vial |
Los viales de bulevirtida se deben conservar en el embalaje original en la nevera (entre 2 y 8 ºC) para proteger bulevirtida de la luz. | Bulevirtida reconstituida se debe utilizar inmediatamente. Las siguientes instrucciones son para disolver una dosis única. | Lávese bien las manos con jabón y agua tibia y séqueselas con una toalla limpia. Una vez que las manos estén limpias, no toque nada más que el medicamento, el material auxiliar y la zona que rodea al lugar de inyección. | Frote la parte superior del vial con un algodón nuevo empapado en alcohol y deje que se seque al aire. Si toca el tapón de goma después de limpiarlo, límpielo de nuevo con otro algodón empapado en alcohol. |
|
|
| |
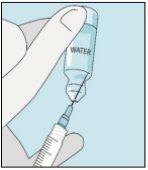
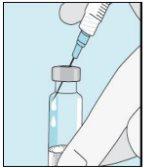
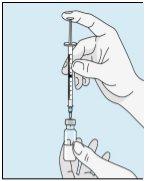
2A Extraer agua estéril | 2B Inyectar agua estéril en el polvo | 2C Mezclar suavemente bulevirtida | |
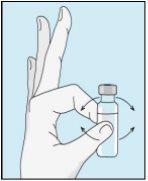
Coja la jeringuilla. Coloque en ella la aguja más larga. ¡Importante!Asegúrese de que la aguja tapada está bien ajustada, presionándola ligeramente mientras la gira en el sentido de las agujas del reloj. Retire el capuchón de plástico. Abra el agua estéril para preparaciones inyectables. Inserte la aguja en el vial e invierta suavemente el vial de agua. Asegúrese de que la punta de la aguja esté siempre por debajo de la superficie del agua para que no entren burbujas de aire en la jeringuilla. Tire lentamente del émbolo hasta tener 1,0 cc/ml de agua estéril dentro de la jeringuilla. Retire cuidadosamente la aguja y la jeringuilla del vial. | Golpee suavemente el vial de bulevirtida para que se suelte el polvo. Introduzca la aguja con agua estéril en el vial de bulevirtida en ángulo. Inyecte el agua estéril lentamente, para que caiga por la pared del vial en el polvo de bulevirtida. | Golpee suavemente el vial de bulevirtida con la punta de los dedos 10 segundos para que el polvo se empiece a disolver. A continuación, gire suavemente el vial de bulevirtida entre las manos para que se mezcle completamente. Asegúrese de que no hay polvo de bulevirtida pegado en las paredes del vial. ¡Importante! No agite el vial de bulevirtida. Si lo agita se formará espuma y el medicamento tardará mucho más tiempo en disolverse. | |
|
| ||
2D Inspeccionar bulevirtida | 2E Bulevirtida lista para la inyección | ||
Cuando el polvo se empiece a disolver, déjelo y se disolverá completamente. Después de darle golpecitos, podría tardar hasta 3 minutos en disolverse. | Cuando esté totalmente mezclada, la solución de bulevirtida debe ser transparente. ¡Importante!Bulevirtida totalmente disuelta debe ser transparente y sin espuma. Si la solución de bulevirtida tiene espuma o es amarillenta, déjela que se disuelva más tiempo. Si ve burbujas, golpee suavemente el vial hasta su desaparición. Si observa partículas en la solución de bulevirtida una vez disuelta (por completo), no utilice ese vial. Consulte al médico o farmacéutico que se lo haya suministrado. |
|
|
|
|
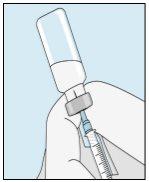
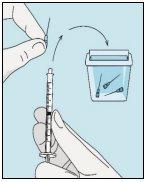
3A Insertar la aguja en vial | 3B Extraer bulevirtida | 3C Terminar la preparación | 3D Cambiar y desechar la aguja |
Coja la jeringuilla. Introduzca la aguja en el vial de bulevirtida líquida. | Invertir suavemente el vial. Asegúrese de que la punta de la aguja esté siempre por debajo de la superficie de la solución de bulevirtida para que no entren burbujas de aire en la jeringuilla. Tire lentamente del émbolo para introducir 1,0 cc/ml de bulevirtida. | Golpee o sacuda suavemente la jeringuilla y empuje/tire del émbolo para eliminar el aire adicional y las burbujas. Para asegurarse de terminar con 1,0 cc/ml de bulevirtida en la jeringuilla, puede que tenga que tirar del émbolo hasta pasada la marca de 1,0 cc/ml. Retire cuidadosamente la aguja y la jeringuilla del vial. | Retire la aguja más larga de la jeringuilla y deséchela correctamente para que nadie se pueda hacer daño. ¡Importante!No vuelva a colocar el capuchón de plástico en la aguja. |
|
|
|
|
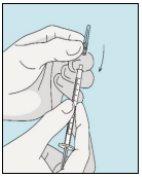
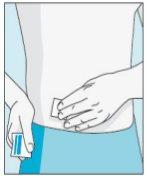
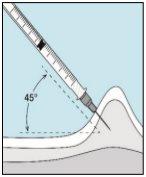
3E Insertar la aguja para la inyección | 3F Elegir el lugar de inyección | 3G Preparar el lugar de inyección | 3H Inyectar bulevirtida |
Colocar la aguja más corta en la jeringuilla. ¡Importante!Asegúrese de que la aguja tapada está bien ajustada, presionándola ligeramente mientras la gira en el sentido de las agujas del reloj. Retire el capuchón de plástico. | Escoja un lugar diferente del que utilizó para su última inyección. Limpie el lugar de inyección con un algodón nuevo empapado en alcohol. Empiece en el centro, aplique presión y limpie con un movimiento circular hacia afuera. ¡Importante!Deje secar la zona al aire. Prepare el vial de bulevirtida. Limpie de nuevo la parte superior del vial de bulevirtida con un algodón nuevo empapado en alcohol. Deje que se seque al aire. | Coja un pellizco de la piel alrededor del lugar de inyección. | Perfore la piel con un ángulo de 45 grados. Se debe introducir la mayor parte de la aguja. Empuje lentamente el émbolo todo su recorrido para inyectar bulevirtida. Retire la aguja de la piel. Retire la aguja de la jeringuilla y deseche ambas adecuadamente para que nadie se pueda hacer daño (ver 3D). |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HEPCLUDEX 2 MG POLVO PARA SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO, 150 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 300 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: INYECTABLE, 90 mgPrincipio activo: enfuvirtideFabricante: Roche Registration GmbhRequiere receta
Médicos online para HEPCLUDEX 2 MG POLVO PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HEPCLUDEX 2 MG POLVO PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes