
HAEMATE P 1200 UI/500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE O PERFUSION.

Cómo usar HAEMATE P 1200 UI/500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE O PERFUSION.
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Haemate P 1200UI FVW/500 UIFVIII
Polvo y disolvente para solución inyectable y para perfusión
Factor von Willebrand humano (FVW)
Factor de coagulación VIII humano (FVIII)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Haemate P y para qué se utiliza
- Qué necesita saber antes de empezar a usar Haemate P
- Cómo usar Haemate P
- Posibles efectos adversos
- Conservación de Haemate P
- Contenido del envase e información adicional
1. Qué es Haemate P y para qué se utiliza
¿Qué es Haemate P?
Haemate P se presenta como polvo acompañado del disolvente. La solución obtenida se administra por inyección o perfusión en vena. Haemate P pertenece a un tipo de medicamentos llamados antihemorrágicos.
Haemate P está fabricado a partir de plasma humano (la parte líquida de la sangre) y contiene factor von Willebrand humano (FVW) y factor de coagulación VIII humano (FVIII).
¿Para qué se utilizaHaemate P?
Como Haemate P contiene tanto FVIII como FVW, es importante saber qué factor necesita más. Si tiene hemofilia A, su médico le recetará Haemate P con el número de unidades de FVIII especificado. Si tiene la enfermedad de von Willebrand, su médico le recetará Haemate P con el número de unidades de FVW especificado.
Enfermedad de von Willebrand
Profilaxis y tratamiento de hemorragias o sangrados quirúrgicos en la enfermedad de von Willebrand, cuando el tratamiento sólo con desmopresina es ineficaz o está contraindicado.
Hemofilia A (deficiencia congénita de factor VIII)
Tratamiento y profilaxis de hemorragias en pacientes con hemofilia A.
Este producto puede ser útil en el manejo de la deficiencia adquirida de factor VIII.
2. Qué necesita saber antes de empezar a usar Haemate P
Las secciones siguientes contienen información que su médico debe tener en cuenta antes de recetarle Haemate P.
No use Haemate P:
- Si es alérgico (hipersensible) al factor von Willebrand humano, al factor de coagulación VIII humano, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Informe a su médico si es alérgico a cualquier medicamento o alimento.
Advertencias y precauciones:
Trazabilidad
Se recomienda encarecidamente que cada vez que se administre Haemate P, su médico deje constancia de la fecha de administración, el número de lote del medicamento para mantener un registro de los lotes utilizados.
Consulte con su médico o farmacéutico antes de empezar a usar Haemate P:
- En caso de que aparezcan reacciones alérgicas o anafilácticas(reacciones alérgicas graves que producen graves dificultades respiratorias o mareos). Es posible que se produzcan reacciones de hipersensibilidad de tipo alérgico. Su médico debe informarle de los primeros síntomas de las reacciones de hipersensibilidad. Entre ellos se incluye ronchas, sarpullido generalizado, presión en el pecho, dificultad en la respiración, caída de la presión sanguínea y anafilaxis (reacciones alérgicas graves que producen graves dificultades respiratorias o mareos). Si se producen esos síntomas, deje de usar el producto de inmediato y póngase en contacto con su médico.
- La formación de inhibidores (anticuerpos), es una complicación conocida que puede producirse durante el tratamiento con todos los medicamentos compuestos por factor VIII. Estos inhibidores, especialmente en grandes cantidades, impiden que el tratamiento funcione correctamente, por lo que se les supervisará cuidadosamente a usted y a su hijo por si desarrollan dichos inhibidores. Si su hemorragia o la de su hijo no se está controlando con Haemate P, consulte a su médico inmediatamente.
- Si padece una enfermedad cardíaca o tiene riesgo de padecerla, informe a su médico o farmacéutico.
- Si para la administración de Haemate P se necesita un dispositivo de acceso venoso central (DAVC), su médico deberá considerar el riesgo de complicaciones relacionadas con el catéter incluyendo las infecciones locales, bacterias en sangre (bacteriemia) y la formación de coágulos de sangre (trombosis) en el sitio de inserción del catéter.
Enfermedad de von Willebrand
- En caso de que exista el riesgo de formación de coágulos de sangre (efectos trombóticos, incluyendo coágulos de sangre en el pulmón), particularmente en el caso de que usted presente factores de riesgo clínicos o de laboratorio (por ejemplo, en periodos perioperatorios sin recibir profilaxis de trombosis, movilización tardía, obesidad, sobredosificación, cáncer), usted deberá ser controlado acerca de los primeros síntomas de trombosis. Debe establecerse la prevención de la trombosis venosa de acuerdo con las recomendaciones actuales. Los pacientes con enfermedad de von Willebrand, especialmente los pacientes con el tipo 3 de esta enfermedad pueden desarrollar anticuerpos neutralizantes (inhibidores) contra el factor de von Willebrand. Su médico le hará las pruebas necesarias para detectar su presencia y considerará si seguir o no con esta terapia.
Su médico tendrá que considerar cuidadosamente los beneficios del tratamiento con Haemate P en comparación con los riesgos de estas complicaciones.
Seguridad viral
Cuando se administran medicamentos derivados del plasma o sangre humanos, no se puede excluir totalmente la aparición de enfermedades debidas a la transmisión de agentes infecciosos. Esto también se refiere a la posible transmisión de patógenos de naturaleza desconocida o emergente o a otros tipos de infecciones. Sin embargo, el riesgo de transmisión de agentes infecciosos se reduce por:
- la selección cuidadosa de los donantes de sangre y plasma para garantizar que se excluye a los posibles portadores de infecciones;
- análisis de cada donación y mezcla de plasmas para detectar signos de virus/infecciones;
- inclusión de etapas en el proceso de fabricación de la sangre o plasma para inactivar o eliminar los virus.
Estos procedimientos se consideran efectivos para virus envueltos como el virus de la inmunodeficiencia humana (VIH, el virus del SIDA), el virus de la hepatitis B y el virus de la hepatitis C (que causan la inflamación del hígado) y para virus no envueltos como el virus de la hepatitis A (inflamación del hígado).
Los procedimientos de inactivación/eliminación pueden tener un valor limitado para virus no envueltos tales como el parvovirus B19.
La infección por parvovirus Bl9 puede ser grave para una mujer embarazada (infección fetal) y para sujetos con inmunodeficiencia o con una producción aumentada de hematíes (por ejemplo, con anemia hemolítica).
Es posible que su médico le recomiende vacunarse contra la hepatitis A y B si recibe de forma regular o repetida concentrados de factor von Willebrand y factor de coagulación VIII humano.
Uso de Haemate P con otros medicamentos
- Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- Este medicamento no debe mezclarse con otros medicamentos, disolventes y diluyentes.
Embarazo, lactancia y fertilidad
- Si está usted embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
- Debido a que la hemofilia A es rara en las mujeres, no se dispone de experiencia relativa al uso de factor VIII durante el embarazo y la lactancia.
- En el caso de la enfermedad de von Willebrand, las mujeres se ven incluso más afectadas que los hombres. Según la experiencia acumulada tras la comercialización, puede recomendarse la sustitución del FVW en la prevención y el tratamiento de hemorragias agudas. No hay estudios clínicos disponibles sobre la terapia sustitutiva con el FVW en mujeres durante el embarazo y la lactancia.
- Durante el embarazo y la lactancia, Haemate P sólo debe administrarse si está claramente indicado.
Conducción y uso de máquinas
Haemate P no afecta a la capacidad para conducir vehículos y usar maquinaria.
Haemate P contiene sodio
Haemate P 1200 UI VWF/500 UI FVIII contiene 26 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada vial. Esto equivale al 1,3% de la ingesta diaria máxima recomendada para un adulto.
3. Cómo usar Haemate P
El tratamiento debe ser iniciado y supervisado por un médico con experiencia en el tratamiento de trastornos de coagulación de la sangre.
Dosificación
La cantidad de factor von Willebrand y factor VIII que necesita y la duración del tratamiento dependen de varios factores, como su peso, la gravedad de la enfermedad, el lugar e intensidad de la hemorragia o la necesidad de evitarla durante una operación o investigación (ver la sección "Esta información está destinada únicamente a profesionales del sector sanitario").
Si le han recetado Haemate P para usarlo en casa, el médico deberá asegurarse de que usted sabe cómo se inyecta y la cantidad que debe utilizar.
Siga exactamente las instrucciones de administración de Haemate P indicadas por su médico o por el personal sanitario del centro de hemofilia.
Si usa más Haemate P del que debe
No se han comunicado síntomas de sobredosis con FVW y FVIII. No obstante, no se puede excluir el riesgo de desarrollar coágulos de sangre (trombosis) en caso de una dosis extremadamente alta, especialmente de productos con FVW que tengan un elevado contenido de FVIII.
Reconstitución y administración
Asegúrese de que trabaja en condiciones estériles en todas las etapas del proceso.
Instrucciones generales
- El polvo liofilizado debe mezclarse (reconstituirse) con el disolvente (líquido) y extraerse del vial en condiciones asépticas.
- La solución debe ser transparente o ligeramente opalescente. Después del filtrado/transvase (consulte más adelante y antes de administrarla al paciente), la solución obtenida debe examinarse visualmente para comprobar que no contiene partículas ni presenta decoloración. Aunque se sigan con precisión las recomendaciones para la preparación de la solución (reconstitución), no es raro que queden algunos residuos o partículas. El filtro incluido en el dispositivo Mix2Vial elimina esas partículas por completo. La filtración no influye en los cálculos de dosificación.
- No use soluciones visiblemente turbias ni soluciones que todavía contengan partículas o residuos después de la filtración.
- Tras la administración, la cantidad de medicamento que no se haya usado y el material residual deben desecharse de acuerdo con la normativa local y siguiendo las instrucciones de su médico.
Reconstitución:
Antes de abrir ninguno de los viales, atempere el polvo de Haemate P y el disolvente acompañante hasta que estén a temperatura ambiente. Para conseguirlo, puede dejar los viales a temperatura ambiente durante aproximadamente una hora o bien puede tenerlos en las manos cerradas durante unos minutos. No exponga los viales al calor directo. Los viales no deben calentarse a una temperatura superior a la del cuerpo (37 °C).
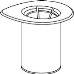
Retire con cuidado las cápsulas protectoras del vial del disolvente y del vial de Haemate P. Limpie la parte exterior de los tapones de goma de ambos viales con una toallita impregnada en alcohol y déjelos secar. Ahora puede transferir el disolvente al vial del polvo con el sistema de administración incluido (Mix2Vial). Por favor, siga las instrucciones siguientes:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transvase de la solución preparada a la jeringa y administración | |
|
|
|
|
Administración
Una vez transferido el producto a la jeringa, debe utilizarse de inmediato.
Para la inyección de Haemate P se recomienda el uso de jeringas desechables de plástico ya que la superficie de vidrio esmerilado de las jeringas que son totalmente de vidrio tiende a pegarse con soluciones de este tipo.
La solución reconstituida debe administrarse lentamente por vía intravenosa a una velocidad no superior a 4 ml por minuto. Tenga cuidado de que no entre sangre en la jeringa llena de producto.
Si tienen que administrarse dosis más elevadas, también puede hacerse por perfusión. Con dicho propósito, transfiera el producto reconstituido a un sistema de perfusión aprobado. La perfusión debe llevarse a cabo siguiendo las instrucciones del médico.
Observe si presenta alguna reacción inmediata. Si se produce alguna reacción que pueda estar relacionada con la administración de Haemate P debe interrumpirse inmediatamente la inyección o perfusión (ver también la sección 2).
Si usa más Haemate P del que debiera
No se han comunicado síntomas de sobredosis con FVW y FVIII. No obstante, no se puede excluir el riesgo de desarrollar coágulos de sangre (trombosis) en caso de una dosis extremadamente alta, especialmente de productos con FVW que tengan un elevado contenido de FVIII.
Si tiene cualquier otra duda sobre el uso del producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos siguientes se han observado muy raramente (en menos de 1 de cada 10.000 pacientes):
- Una reacción alérgica repentina (como angioedema, quemazón o picor en el punto de perfusión, escalofríos, sofocos, urticaria generalizada, dolor de cabeza, ronchas, hipotensión, letargo, náuseas, inquietud, taquicardia, presión en el pecho, sensación de hormigueo, vómito o respiración dificultosa) y en algunos casos puede progresar a anafilaxia grave (incluyendo shock).
- Incremento de la temperatura corporal (fiebre).
Enfermedad de von Willebrand
- Muy raramente, hay riesgo de acontecimientos trombóticos o tromboembólicos incluyendo coágulos de sangre en el pulmón (riesgo de formación y migración de coágulos de sangre en el sistema vascular, formado por las arterias y venas, con un impacto potencial en los órganos).
- En pacientes que reciben productos con FVW, unos niveles plasmáticos excesivos de FVIII:C de forma sostenida pueden aumentar el riesgo de formación de coágulos sanguíneos (consulte también la sección 2).
- Los pacientes con enfermedad de von Willebrand pueden desarrollar muy raramente inhibidores (anticuerpos neutralizadores) del FVW. Si aparecen dichos inhibidores, se manifiesta una ausencia de respuesta clínica que conduce a un sangrado continuo. Esto ocurre especialmente en pacientes con una forma específica de la enfermedad de von Willebrand, llamada de tipo 3. Esos anticuerpos precipitan y pueden aparecer al mismo tiempo que las reacciones anafilácticas. Por lo tanto, debe evaluarse la presencia de un inhibidor en los pacientes que experimentan reacción anafiláctica. En tales casos, se recomienda ponerse en contacto con un centro especializado en hemofilia.
Hemofilia A
- En los niños que no han recibido tratamiento previo con medicamentos compuestos por factor VIII pueden producirse anticuerpos inhibidores (ver sección 2) muy frecuentemente (más de 1 de cada 10 pacientes); sin embargo, en los pacientes que han recibido tratamiento previo con factor VIII (más de 150 días de tratamiento), el riesgo es poco frecuente (menos de 1 de cada 100 pacientes). Si esto sucede, los medicamentos que toman usted o su hijo pueden dejar de funcionar correctamente y usted o su hijo pueden sufrir una hemorragia persistente. En ese caso, contacte con su médico inmediatamente.
Otros efectos adversos en niños y adolescentes
Se espera que la frecuencia, el tipo y la gravedad de los efectos adversos en niños sea el mismo que en adultos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es.
Para información sobre la seguridad viral, ver “Advertencias y precauciones” (sección 2).
5. Conservación de Haemate P
- Mantener este medicamento fuera de la vista y del alcance de los niños
- No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de EXP. La fecha de caducidad es el último día del mes que se indica.
- Conservar por debajo de 25 ºC.
- No congelar.
- Mantener el vial en el embalaje exterior para protegerlo de la luz.
- Haemate P no contiene conservantes, por ello, la solución preparada debe usarse inmediatamente.
- Si la solución preparada no se administra inmediatamente, debe usarse en un periodo de 3 horas.
- Una vez transferido el producto a la jeringa, debe utilizarse de inmediato.
Los medicamentos no se deben tirar por los desagües, ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Haemate P
Los principios activos son: Factor von Willebrand humano y factor de coagulación VIII humano.
Los demás componentes son: Albúmina humana, glicina, cloruro sódico, citrato sódico, hidróxido de sodio o ácido clorhídrico (en pequeñas cantidades para ajustar el pH).
Disolvente:agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Haemate P se presenta como un polvo blanco o amarillo pálido o sólido friable y se suministra con agua para preparaciones inyectables como disolvente. La solución preparada debe ser transparente o ligeramente opalescente, es decir, podría brillar al ponerla a contraluz, pero no debe contener ningún tipo de partículas detectables.
Presentación
Caja que contiene:
1 vial con polvo
1 vial con 10 ml de agua para preparaciones inyectables
1 trasvasador con filtro 20/20
Equipo de administración (caja interior):
1 jeringa de 10 ml de un solo uso
1 equipo de punción venosa
2 toallitas con alcohol
1 apósito adhesivo no estéril
Titular de la autorización de comercialización y responsable de la fabricación
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Barcelona (España)
Responsable de la fabricación
CSL Behring, GmbH
Emil-von-Behring-Str. 76
35041 Marburg (Alemania)
Fecha de la última revisión de este prospecto: Julio 2023
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
-------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Posología
Enfermedad de von Willebrand
Es importante calcular la dosis utilizando el número de UI de FVW:RCo especificado.
Por lo general, la administración de 1 UI de FVW:RCo/kg de peso corporal incrementa los niveles circulantes de VWF:RCo en 0,02 UI/ml, lo que representa un aumento del 2%.
Deben alcanzarse niveles superiores a 0,6 UI de FVW:RCo/ml (60%) y niveles superiores a 0,4 UI de FVIII:C/ml (40%).
Las dosis recomendadas para conseguir la hemostasia son 40 - 80 UI de FVW:RCo/kg de peso y 20 - 40 UI de FVIII:C/kg de peso.
En ciertos casos puede requerirse una dosis inicial de 80 UI de factor de von Willebrand, especialmente en aquellos pacientes con una enfermedad de von Willebrand Tipo 3, en los que la terapia de mantenimiento de niveles adecuados puede requerir dosis superiores a las que demandan los otros tipos de enfermedad de von Willebrand.
Prevención de hemorragias en casos de cirugía o lesiones graves:
Para prevenir sangrados profusos durante o después de cirugía, la administración del producto debe iniciarse de 1 a 2 horas antes de comenzar la cirugía.
Una dosis adecuada debe repetirse a intervalos de 12 a 24 horas. La dosis y la duración del tratamiento dependen del estado clínico del paciente, del tipo y gravedad del sangrado y de los niveles de FVW:RCo y FVIII:C.
Cuando se administran productos que contienen factor VIII y factor de von Willebrand, el médico responsable del tratamiento debe tener en cuenta que el tratamiento continuado puede ocasionar un incremento excesivo del FVIII:C. Después de un tratamiento de 24 - 48 horas, y a fin de evitar un incremento indeseable del FVIII:C, deberá considerarse una reducción de las dosis y/o un aumento de los intervalos entre administraciones, o el uso de productos de factor de von Willebrand que contengan bajos niveles de factor VIII.
Población pediátrica
La posología en pediatría se basa en el peso corporal y por lo tanto sigue, generalmente, las mismas directrices que se usan para los adultos. La frecuencia de administración debe estar siempre orientada a conseguir la eficacia clínica en cada caso particular.
Hemofilia A
Monitorización del tratamiento
Durante el curso del tratamiento se recomienda controlar adecuadamente los niveles de factor VIII para determinar la dosis a administrar y la frecuencia de las perfusiones repetidas. La respuesta individual de los pacientes a la terapia con factor VIII puede variar, alcanzándose diferentes niveles de recuperación in vivoy demostrando distintas semividas. La dosis basada en el peso corporal puede requerir un ajuste en pacientes con bajo peso o sobrepeso.En el caso de intervenciones de cirugía mayor, es indispensable monitorizar con precisión la terapia de sustitución mediante pruebas de la coagulación (actividad plasmática del factor VIII).
Debe monitorizarse en los pacientes el desarrollo de inhibidores del factor VIII. Ver también sección 2.
La dosis y duración del tratamiento dependen del grado de la deficiencia de factor VIII, de la localización y gravedad de la hemorragia y del estado clínico del paciente.
Es importante calcular la dosis utilizando el número de UI de FVIII:C especificado.
El número de unidades de factor VIII administradas se expresa en Unidades Internacionales (UI), en relación con el estándar actual de la Organización Mundial de la Salud (OMS) vigente para concentrados de factor VIII. La actividad plasmática de factor VIII se expresa como un porcentaje (en relación con el plasma humano normal) o preferiblemente en Unidades Internacionales (en relación con un estándar internacional para factor VIII en plasma).
La actividad de una unidad internacional de factor VIII es equivalente a la cantidad del factor VIII contenido en un ml de plasma humano normal.
Tratamiento a demanda
El cálculo de la dosis necesaria de factor VIII se basa en la observación empírica de que 1 UI de factor VIII por kg de peso corporal eleva la actividad plasmática de factor VIII en aproximadamente un 2% (2 UI/dl). La dosis necesaria se determina mediante la fórmula siguiente:
Unidades necesarias = peso corporal (kg) x aumento deseado de factor VIII (% o UI/dl) x 0,5
La dosis y la frecuencia de la administración se establecerán siempre en función de la eficacia clínica observada en cada caso.
En el caso de episodios hemorrágicos como los detallados a continuación, la actividad de factor VIII no debe ser inferior al nivel plasmático de actividad establecido (en % de plasma normal o UI/dl) en el período correspondiente. Puede emplearse la siguiente tabla como guía de dosificación en episodios hemorrágicos y cirugía:
Grado de hemorragia/tipo de cirugía | Nivel de factor VIII requerido (% o UI/dl) | Frecuencia de dosis (horas)/duración de la terapia (días) |
Hemorragia | ||
Hemartrosis precoz, sangrado muscular o de la cavidad bucal | 20 - 40 | Repetir cada 12 - 24 horas. Al menos 1 día, hasta que la hemorragia se haya resuelto, en función del dolor, o hasta la cicatrización adecuada de la herida. |
Hemartrosis más extensa, sangrado muscular o hematoma | 30 - 60 | Repetir la perfusión cada 12 - 24 horas, durante 3 - 4 días o más hasta que el dolor y la discapacidad aguda se hayan resuelto. |
Hemorragias con riesgo vital | 60 - 100 | Repetir la perfusión cada 8 - 24 horas hasta que desaparezca el riesgo. |
Cirugía | ||
Menorincluyendo la extracción dental | 30 - 60 | Cada 24 horas, al menos 1 día, hasta la cicatrización de la herida. |
Mayor | 80 - 100(pre y posoperatorio) | Repetir la perfusión cada 8 - 24 horas hasta la adecuada cicatrización de la herida, y luego terapia durante un mínimo de 7 días para mantener una actividad de factor VIII del 30% - 60% (UI/dl). |
Profilaxis
Para la profilaxis a largo plazo en pacientes con hemofilia A grave, las dosis usuales son de 20 a 40 UI de factor VIII por kg de peso corporal, a intervalos de 2 o 3 días. En ciertos casos, especialmente en pacientes jóvenes, puede ser necesario acortar los intervalos entre administraciones o utilizar dosis más elevadas.
Población pediátrica
No se dispone de experiencia clínica en el tratamiento de niños con Haemate P.
Advertencias y precauciones especiales de uso
Cuando se utiliza un producto que contiene el factor de von Willebrand, el médico responsable del tratamiento debe tener en cuenta que un tratamiento continuado puede causar un aumento excesivo de FVIII:C. En pacientes que reciban productos con FVW que contienen FVIII, es necesario supervisar los niveles plasmáticos de FVIII:C para evitar niveles plasmáticos excesivos de forma sostenida, lo que podría aumentar el riesgo de efectos trombóticos, y es necesario evaluar medidas antitrombóticas.
Reacciones adversas
Cuando se necesitan dosis muy grandes o frecuentemente repetidas, cuando hay presencia de inhibidores o cuando hay implicados cuidados pre o posquirúrgicos, debe supervisarse la aparición de síntomas de hipervolemia en todos los pacientes. Además, en pacientes con grupos sanguíneos A, B y AB debe controlarse si hay síntomas de hemólisis intravascular y/o disminución de los valores de hematocrito.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HAEMATE P 1200 UI/500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE O PERFUSION.Forma farmacéutica: INYECTABLE, 100 U.I. FVIII/ 120 U.I. FVW por mlPrincipio activo: Von Willebrand factor and coagulation factor VIII in combinationFabricante: Instituto Grifols S.A.Requiere recetaForma farmacéutica: INYECTABLE, 25 U.I. FVIII/ 30 U.I. FVW por mlPrincipio activo: Von Willebrand factor and coagulation factor VIII in combinationFabricante: Instituto Grifols S.A.Requiere recetaForma farmacéutica: INYECTABLE, 50 U.I. FVIII/60 UI FVW por mlPrincipio activo: Von Willebrand factor and coagulation factor VIII in combinationFabricante: Instituto Grifols S.A.Requiere receta
Médicos online para HAEMATE P 1200 UI/500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE O PERFUSION.
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HAEMATE P 1200 UI/500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE O PERFUSION., sujeto a valoración médica y a la normativa local.
Preguntas frecuentes




 1
1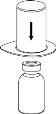 2
2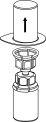 3
3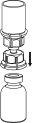 4
4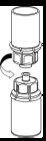 5
5 6
6 7
7 8
8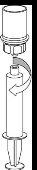 9
9










