
GAMAVAN 360 MICROGRAMOS/ML SOLUCION ORAL

Cómo usar GAMAVAN 360 MICROGRAMOS/ML SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Gamavan 360 microgramos/ml solución oral y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Gamavan 360 microgramos/ml solución oral
- Cómo tomar Gamavan 360 microgramos/ml solución oral
- Posibles efectos adversos
- Conservación de Gamavan 360 microgramos/ml solución oral
- Contenido del envase e información adicional
Introducción
Prospecto: Información para el paciente
Gamavan 360microgramos/ml solución oral
(desmopresina)
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted o a su hijo y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted o su hijo, ya que puede perjudicarles.
- Si usted o su hijo experimentan efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Gamavan 360 microgramos/ml solución oral y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Gamavan 360 microgramos/ml solución oral
- Cómo tomar Gamavan 360 microgramos/ml solución oral
- Posibles efectos adversos
- Conservación de Gamavan 360 microgramos/ml solución oral
- Contenido del envase e información adicional
1. Qué es Gamavan 360 microgramos/ml solución oral y para qué se utiliza
El principio activo de este medicamento se llama desmopresina. La desmopresina es muy parecida a una sustancia que se produce de forma natural en el organismo (la hormona hipofisaria vasopresina), que reduce temporalmente la cantidad de orina producida por el organismo. Este medicamento está destinado solamente para uso oral.
Desmopresina sirve para tratar:
- La diabetes insípida central, una enfermedad que se caracteriza por provocar una sed extrema y una producción continua de grandes volúmenes de orina muy diluida a consecuencia de la producción insuficiente de la hormona vasopresina.
- Enuresis nocturna primaria (incontinencia urinaria durante la noche) en pacientes mayores de 5 años con capacidad normal de concentrar la orina.
2. Qué necesita saber antes de empezar a tomar Gamavan 360 microgramos/ml solución oral
No tome Gamavan:
- si es alérgico(hipersensible) a la desmopresinao a alguno de los demás componentesde este medicamento (incluidos en la sección 6),
- si bebede manera excepcional grandes cantidadesde líquido (presenta polidipsia habitual o psicógena),
- si toma medicamentos que aumentan la producción de orina(diuréticos),
- si padece problemas de corazón,
- si sufre predisposición o padece una deficiencia de sodioen la sangre (hiponatremia), incapacidad para reducir la ingesta de líquidos, por ejemplo, en trastornos de la memoria,
- si presenta insuficiencia renal,
- si padece el “síndrome de secreción inadecuada de hormona antidiurética” (SIHAD).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Gamavan
Cuando esté tomando desmopresina, evite la ingesta excesiva de líquidos dado que puede provocar la retención de líquidos en el cuerpo y/o una disminución de sodio en la sangre, con o sin efectos secundarios (ver sección 4. Posibles efectos adversos).
Se debe prestar especial cuidado con desmopresina para evitar la retención de líquidos en el cuerpo y la disminución del sodio en la sangre en los siguientes casos:
- Si es una persona de edad avanzada
- Si presenta algún problema médico que cause un desequilibrio de agua y/o electrolitosen el organismo, como infección, fiebre o malestar estomacal Si padece un problema grave de vejiga o una alteración de la evacuación de la orina
- Si tiene asma, epilepsiay migrañas
- Si padece insuficiencia renal y/o una enfermedad cardiovascular. En caso de insuficiencia renal crónica, el efecto antidiurético de Gamavan sería inferior al habitual.
Otros medicamentos y Gamavan
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, especialmente:
- antidepresivos tricíclicos o ISRS(utilizados para tratar la depresión),
- carbamazepina(utilizada para tratar la epilepsia),
- clorpromazina(utilizada para tratar la psicosis o la esquizofrenia),
- medicamentos para el dolor y/o la inflamacióndenominados fármacos antiinflamatorios no esteroideos (AINE), tales como indometacina, ibuprofeno o ácido acetilsalicílico,
- loperamida(utilizada para tratar la diarrea),
- diuréticos.
Estos medicamentos aumentan el riesgo de retención de líquidos, lo que diluye la sal en el organismo.
- La dimeticona (utilizada en el tratamiento de los gases intestinales), debido a que disminuye la absorción de la desmopresina.
Toma de Gamavan con alimentos y bebidas
Ver sección 3 “Cómo tomar”.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Es preferible evitar la toma de Gamavan durante el embarazo.
La desmopresina pasa a la leche materna. Si va a tratarse con desmopresina, debería dejar la lactancia materna.
Conducción y uso de máquinas
Gamavan no afecta a la capacidad para conducir o utilizar máquinas.
Información importante sobre algunos de los componentes de Gamavan:
Este medicamento puede producir reacciones alérgicas (posiblemente retardadas) porque contiene metil-parahidroxibenzoato sódico y propil-parahidroxibenzoato sódico.
Este medicamento contiene menos de 23 mg de sodio (1mmol) por ml; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Gamavan 360 microgramos/ml solución oral
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
En dosis bajas, Gamavan puede verse afectada por la ingesta de alimentos. Si nota que Gamavan es menos eficaz, deberá tomar el medicamento sin comida antes de aumentar la dosis.
Cuando tome este medicamento para la incontinencia urinaria durante la noche, reduzca al mínimo el consumo de líquidosdesde 1hora antesde tomar la dosis de Gamavan y hasta 8horas después.
Tratamiento de la diabetes insípida central:
Adultos y niños:Su médico le recetará la dosis más conveniente en su caso. La dosis inicialrecomendada es de 0,25 ml (90 microgramos) tres veces al día. Posteriormente, el médico ajustará la dosis según la respuesta de cada paciente. La dosis diariarecomendadaes entre 0,5 ml (180 microgramos) a 3 ml (1080 microgramos) deGamavan. La dosis de mantenimientovaría entre 0,25 y 0,5 ml (90 – 180 microgramos) de Gamavan tres veces al día.
Es importante señalar si aparecen síntomas de retención de líquidos en el cuerpo y/o disminución de sodio en la sangre (ver sección 4: Posibles efectos adversos), en cuyo caso debe interrumpirse el tratamiento para cambiar la dosis.
Enuresis nocturna primaria (incontinencia urinaria durante la noche) en pacientes mayores de 5años:
Adultos y niños:La dosis inicial recomendada es de 0,5 ml (180 microgramos) de Gamavan una hora antes de acostarse. La dosis puede aumentarse hasta 1 ml (360 microgramos) de Gamavan si la dosis inicial no es suficiente. La necesidad de continuar el tratamiento se comprueba normalmente cada tres meses introduciendo un período sin tratamiento de al menos una semana.
Instrucciones de uso:
- Abrir el frasco (en la primera apertura se rompe el precinto).

- Insertar la jeringuilla oral en el dispensador y dar la vuelta al frasco para llenarlo con la dosis que se va a administrar.
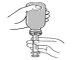
- Sacar la jeringuilla oral del frasco y comprobar que el fondo de la jeringuilla presenta la cantidad correcta.
- Poner la jeringuilla en la boca y administrar la dosis.
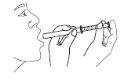
- Enjuagar con agua después de cada uso y cerrar el frasco. Conservar el frasco en el embalaje exterior para protegerlo de la luz.
Si toma más Gamavan del que debe
Si toma más Gamavandel que le han recetado, póngase en contacto con su médico o farmacéutico inmediatamente.
Una sobredosis puede prolongar el efecto de la desmopresina y aumentar el riesgo de que se acumule agua en el cuerpo o que descienda el nivel de sodio en la sangre. Los síntomas pueden incluir dolor de cabeza, náuseas y vómitos, aumento de peso y, en casos graves, convulsiones. Se recomienda interrumpir el tratamiento, restringir la ingesta de líquidos y administrar un tratamiento sintomático en caso necesario.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Gamavan
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Gamavan
No deje de tomar Gamavan antes de finalizar el tratamiento, ya que puede no tener el efecto esperado. Solo debe cambiar o interrumpir el tratamiento si se lo indica su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta alguno de los síntomas siguientes, deje de tomar Gamavan y consulte a un médico o acuda de inmediato a un hospital:
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- síntomas de retención de líquidosen el cuerpo, como dolor de cabeza particularmente fuerte o prolongado, vértigos, sentirse o estar enfermo, aumento de peso y, en casos graves, convulsiones o inconsciencia (hiponatremia)
- reacciones alérgicascomo erupción, picor, fiebre, hinchazón de los labios, la cara, la garganta o la lengua que causa dificultades para tragar o respirar
- síntomas de una acumulación de sodio en el cuerpo como confusión, contracciones musculares o espasmos (hipernatremia)
Informe a su médico o farmacéuticosi observa alguno de estos otros efectos adversos:
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- dolor abdominal, náuseas, presión arterial alta, diarrea, estreñimiento, problemas de vejiga y riñones, hinchazón,
- dolor de cabeza
Poco frecuentes(pueden afectar hasta 1de cada 100personas)
- cambios de humor repentinos y exagerados, agresividad, problemas de vejiga y riñones, hinchazón de manos y pies, fatiga
Raros (pueden afectar hasta 1 de cada 1.000 personas)
- ansiedad, pesadillas, cambios de humor, somnolencia, presión arterial alta, irritabilidad
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- inconsciencia, debilidad, convulsiones, trastornos emocionales, comportamiento anómalo, depresión, alucinaciones (ver, oír o sentir cosas que no están presentes), insomnio, trastornos de la atención, inquietud, hemorragias nasales, sudoración, erupciones cutáneas, urticaria, alergias cutáneas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Gamavan 360 microgramos/ml solución oral
Mantener este medicamento fuera de la vista y del alcance de los niños.
Este medicamento no requiere condiciones especiales de conservación.
Conservar en el embalaje original.
Tras la primera apertura, conservar por debajo de 25 °C durante un plazo máximo de 8 semanas.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase o en la etiqueta después de CAD o EXP. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Gamavan
- El principio activo es desmopresina. Cada ml de la solución oral contiene 360 microgramos de desmopresina (en forma de acetato).
- Los demás componentes son:
- metil-parahidroxibenzoato sódico (E‑219)
- propil parahidroxibenzoato sódico (E‑217)
- ácido clorhídrico (para ajuste del pH)
- agua purificada
Aspecto del producto y contenido del envase
Gamavanes una solución transparente incluida en una botella de vidrio ámbar tapada con un dispensador de plástico, provisto de un tapón de rosca también de plástico. El frasco contiene 15 ml de solución. El frasco viene embalado en una caja de cartón con una jeringuilla de dosificación de 1,5 ml. La jeringuilla está graduada de 0 a 1,5 ml con divisiones cada 0,1 ml. La graduación correspondiente a las dosis de 0,25 ml, 0,5 ml y 1,0 ml está marcada específicamente.
Titular de la autorización de comercialización
GP-Pharm, S.A.
Polígono Industrial Els Vinyets – Els Fogars, sector 2
Carretera Comarcal C244, Km 22,
08777 – Sant Quintí de Mediona (Barcelona) ESPAÑA
Responsable de la fabricación
Laboratorio Reig Jofré S.A.,
Gran Capitàn nº 10,
08970 Sant Joan Despí – Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Francia | NIWINUR 360 microgrammes/ml, solution orale |
España | Gamavan 360 microgramos/ml solución oral |
Fecha de la última revisión de este prospecto: Mayo 2021
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GAMAVAN 360 MICROGRAMOS/ML SOLUCION ORALForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 360mcg/ml desmopresina(base anhidra)Principio activo: DesmopresinaFabricante: Laboratorio Reig Jofre, S.A.Requiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 120 microgramosPrincipio activo: DesmopresinaFabricante: Aristo Pharma GmbhRequiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 240 microgramosPrincipio activo: DesmopresinaFabricante: Aristo Pharma GmbhRequiere receta
Médicos online para GAMAVAN 360 MICROGRAMOS/ML SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GAMAVAN 360 MICROGRAMOS/ML SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















