
FYREMADEL 0,25 MG / 0,5 ML SOLUCION INYECTABLE EN JERINGA PRECARGADA EFG


Cómo usar FYREMADEL 0,25 MG / 0,5 ML SOLUCION INYECTABLE EN JERINGA PRECARGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fyremadel0,25 mg/0,5 ml solución inyectable en jeringa precargada EFG
Ganirelix
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fyremadel y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fyremadel
- Cómo usar Fyremadel
- Posibles efectos adversos
- Conservación de Fyremadel
Contenido del envase e información adicional
1. Qué es Fyremadel y para qué se utiliza
Fyremadelcontiene el principio activo ganirelix y pertenece a un grupo de medicamentos denominados “antagonistas de la hormona liberadora de gonadotropinas“que actúa contra la acción de la hormona liberadora de gonadotropinas (GnRH) endógena. La GnRH regula la liberación de gonadotropinas (hormona luteinizante (LH) y hormona foliculoestimulante (FSH)). Las gonadotropinas desempeñan un importante papel en la fertilidad y reproducción humanas. La FSH es necesaria en las mujeres para el crecimiento y desarrollo de los folículos en los ovarios. Los folículos son pequeñas vesículas redondeadas que contienen los óvulos. La LH es necesaria para que los óvulos maduros se liberen de los folículos de los ovarios (es decir, la ovulación). Fyremadelinhibe la acción de la GnRH, lo que causa la supresión de la liberación especialmente de la LH.
Para qué se utiliza Fyremadel
En las mujeres en tratamiento con técnicas de reproducción asistida, como la fecundación in vitro (FIV) y otros métodos, ocasionalmente puede producirse la ovulación prematura, lo que causa una reducción significativa de la probabilidad de quedarse embarazada.Fyremadelse utiliza para prevenir la liberación prematura de LH, que puede causar una ovulación prematura.
En los estudios clínicos se utilizó Fyremadelcon hormona foliculoestimulante (FSH) recombinante o con corifolitropina alfa, un estimulante folicular de acción prolongada.
2. Qué necesita saber antes de empezar a usar Fyremadel
No useFyremadel
- si es alérgico a ganirelix o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si es hipersensible a la hormona liberadora de gonadotropinas (GnRH) o a sus análogos
- si padece una enfermedad moderada o grave del riñón o del hígado;
- si está embarazada o en período de lactancia.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Fyremadel:
- Si tiene una alergia activa, comuníqueselo a su médico. Su médico decidirá dependiendo de la gravedad si se necesitan controles adicionales durante el tratamiento. Se han observado casos de reacciones alérgicas, incluso tras la primera dosis
- Se han notificado reacciones alérgicas, tanto generalizadas como locales, incluyendo habones (urticaria), hinchazón de la cara, labios, lengua y/o garganta que pueden provocar dificultad para respirar y/o tragar (angioedema y/o anafilaxia) (ver también sección 4). Si tiene una reacción alérgica, deje de utilizar Fyremadel y busque asistencia médica inmediatamente.
- Alergia al látex: el protector de la aguja contiene goma natural seca/látex que puede causar reacciones alérgicas al estar en contacto con la aguja.
- Durante o después de la estimulación hormonal de los ovarios puede desarrollarse el síndrome de hiperestimulación ovárica. Este síndrome está relacionado con el procedimiento de estimulación con gonadotropinas. Le recomendamos que lea el prospecto del medicamento con gonadotropina que le hayan recetado
- La incidencia de malformaciones congénitas tras el uso de técnicas de reproducción asistida puede ser ligeramente superior que tras concepciones espontáneas. Se considera que esta incidencia ligeramente superior está relacionada con las características de los pacientes que siguen tratamientos de fertilidad (por ejemplo, edad de la mujer, características del semen) y con la mayor incidencia de embarazos múltiples registrada tras el uso de técnicas de reproducción asistida. La incidencia de malformaciones congénitas tras el uso de técnicas de reproducción asistida con Fyremadel no es diferente de la incidencia con el uso de otros análogos de la GnRH, en técnicas de reproducción asistida
- Existe un ligero aumento del riesgo de un embarazo fuera del útero (un embarazo ectópico) en mujeres con las trompas de Falopio dañadas
- No se ha establecido la eficacia y seguridad de Fyremadel en mujeres que pesan menos de 50 kg o más de 90 kg. Consulte a su médico para más información.
Niños y adolescentes
No es apropiado el uso de Fyremadel en niños o adolescentes.
Uso deFyremadelcon otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Fyremadel debe utilizarse durante la estimulación ovárica controlada para técnicas de reproducción asistida (TRA). No use Fyremadel durante el embarazo y la lactancia.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se han estudiado los efectos sobre la capacidad para conducir y utilizar máquinas.
Fyremadelcontiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por inyección, esto es esencialmente "exento de sodio".
3. Cómo usar Fyremadel
Fyremadel se utiliza como parte del tratamiento en las técnicas de reproducción asistida (TRA), incluyendo la fecundación in-vitro (FIV).
Usted mismo se administrará las inyecciones por tanto, su médico le debe explicar cómo hacerlo. Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Fase 1
La estimulación ovárica con hormona foliculoestimulante (FSH) o con corifolitropina puede empezar al segundo o tercer día de su menstruación.
Fase 2
Debe inyectarse el contenido de la jeringa de Fyremadel (0,25 mg) una vez al día justo bajo la piel, empezando el quinto o el sexto día de la estimulación. Según sea su respuesta ovárica, su médico puede decidir empezar otro día.
Fyremadel y la FSH deben administrarse aproximadamente al mismo tiempo. Sin embargo, estos medicamentos no deben mezclarse y se deben inyectar en diferentes lugares.
El tratamiento diario con Fyremadel debe continuarse hasta que existan suficientes folículos de tamaño adecuado.
Fase 3
La maduración final de los óvulos en los folículos puede inducirse administrando gonadotropina coriónica humana (hCG). El tiempo transcurrido entre dos inyecciones de Fyremadel y entre la última inyección de Fyremadel y la inyección de hCG no debe exceder las 30 horas, en caso contrario, puede presentarse una ovulación prematura (es decir, liberación de los óvulos). Por tanto, si la inyección de Fyremadel es por la mañana, el tratamiento con Fyremadel debe mantenerse durante todo el período de tratamiento con gonadotropina, incluyendo el día en que se induce la ovulación. Si la inyección de Fyremadel es por la tarde, la última inyección de Fyremadel debe administrarse la tarde de la víspera del día en que se induce la ovulación.
Instrucciones de uso
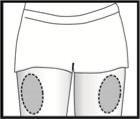
Lugar de la inyección
Fyremadel se presenta en jeringas precargadas que contienen una dosis. El contenido de la jeringa debe inyectarse lentamente justo bajo la piel, preferiblemente en el muslo. Compruebe la solución antes de usar. No use la solución si contiene partículas o no es transparente. Si se administra las inyecciones usted misma o su pareja, siga cuidadosamente las instrucciones que aparecen a continuación. No mezcle Fyremadel con otros medicamentos.
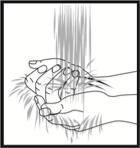
Preparación del lugar de la inyección
Lávese las manos minuciosamente con agua y jabón. El lugar de la inyección debe limpiarse con un desinfectante (por ejemplo alcohol) para eliminar las bacterias de la superficie. Limpie unos 5 cm alrededor del punto donde se pinchará y deje secar el desinfectante al menos durante un minuto antes de inyectar.
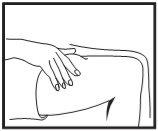
Inserción de la aguja
Retire el capuchón de la aguja. Pellizque un área extensa de piel entre los dedos índice y pulgar. Inserte la aguja en la base del lugar donde ha pellizcado la piel en un ángulo de 45º con respecto a la superficie de la piel. Debe variarse el lugar de la inyección en cada inyección.
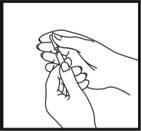
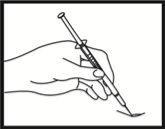
Comprobación de la posición correcta de la aguja
Retire suavemente el émbolo para comprobar si la aguja está colocada correctamente. Si entra sangre en la jeringa, significa que la punta de la aguja ha penetrado en un vaso sanguíneo. Si esto ocurre, no inyecte Fyremadel, sino que retire la jeringa, cubra el lugar de la inyección con una torunda con desinfectante y presione; debe dejar de sangrar al cabo de uno o dos minutos. No utilice esta jeringa y elimínela adecuadamente. Empiece otra vez con una jeringa nueva.
Inyección de la solución
Una vez se ha colocado la aguja correctamente, presione el émbolo lenta y constantemente para inyectar la solución correctamente y que los tejidos de la piel no se dañen.
Extracción de la jeringa
Retire la jeringa rápidamente y presione en el lugar de la inyección con una torunda con desinfectante.
Use la jeringa precargada sólo una vez.
Si usa másFyremadeldel que debe
Consulte a su médico. En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usarFyremadel
Si se da cuenta de que ha olvidado inyectarse una dosis, adminístresela lo antes posible.
No tome una dosis doble para compensar las dosis olvidadas.
Si se retrasa más de 6 horas (por tanto, el intervalo entre dos inyecciones se prolonga más de 30 horas), adminístrese la dosis lo antes posible yacuda a su médico para que le aconseje.
Si interrumpe el tratamiento conFyremadel
No deje de usar Fyremadel salvo que lo indique su médico, ya que esto puede afectar el resultado de su tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes (pueden afectar hasta mas de 1 de cada 10 mujeres)
- reacciones locales en la piel en el lugar de la inyección (principalmente enrojecimiento, con o sin hinchazón). La reacción local normalmente desaparece en un plazo de 4 horas tras la administración.
Poco frecuentes (pueden afectar hasta 1 de cada 100 mujeres)
- dolor de cabeza
- náuseas
- malestar (sensación general de estar enfermo, sentirse mal).
Muy raros (pueden afectar hasta 1 de cada 10.000 mujeres)
- se han observado, incluso tras la primera dosis, reacciones alérgicas.erupción
- hinchazón facial
- dificultad para respirar (disnea)
- hinchazón de cara, labios, lengua y/o garganta, que pueden provocar dificultad para respirar y/o tragar (angioedema y/o anafilaxia)
- habones (urticaria).
Además, se han observado efectos adversos relacionados con el tratamiento de hiperestimulación ovárica controlada, por ejemplo:
- dolor abdominal,
- síndrome de hiperestimulación ovárica (SHO), (SHO ocurre cuando los ovarios reaccionan de forma exagerada a los medicamentos para la fertilidad que está tomando)
- embarazo ectópico (cuando el embrión se desarrolla fuera del útero)
- aborto (ver el prospecto del medicamento con FSH que esté usando).
Después de la primera dosis de Fyremadel se ha comunicado el empeoramiento de un eczema que ya presentaba una paciente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fyremadel
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de ‘CAD’. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Inspeccionar la jeringa antes de usar. La jeringa sólo debe usarse si la solución es transparente y sin partículas y el envase no está dañado.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deFyremadel
- El principio activo es ganirelix. Cada jeringa precargada contiene 0,25 mg de ganirelix (como acetato) en 0,5 ml de solución acuosa.
- Los demás componentes son ácido acético glacial (E260), manitol (E421) y agua para preparaciones inyectables. Puede haberse ajustado el pH (medida de acidez) con hidróxido de sodio y ácido acético glacial.
Aspecto del producto y contenido del envase
Fyremadel es una solución acuosa inyectable transparente e incolora. La solución está lista para usarse, administrándola por vía subcutánea. El protector de la aguja contiene goma natural seca/látex, que está en contacto con la aguja.
Fyremadel se presenta en envases de 1 ó 5 jeringas precargadas con agujas de inyección (27 G).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
FERRING S.A.U.
C/Arquitecto Sánchez Arcas nº 3 1º28040,Madrid-España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria: Fyremadel 0,25 mg/0,5 ml Injektionslösung in einer Fertigspritze
Dinamarca: Fyremadel 0,25 mg/0,5 ml injektionsvæske, opløsning i fyldt injektionssprøjte
España: Fyremadel 0,25 mg/0,5 ml solución inyectable en jeringa precargada EFG
Finlandia: Fyremadel 0,25 mg/0,5 ml injektioneste, liuos esitäytetty ruisku
Francia: Fyremadel 0,25 mg/0,5 ml solution injectable en seringue pré-remplie
Alemania: Fyremadel 0,25 mg/0,5 ml Injektionslösung in einer Fertigspritze
Italia: Fyremadel 0,25 mg/0,5 ml soluzione iniettabile in siringa preriempita
Países Bajos: Fyremadel 0,25 mg/0,5 ml oplossing voor injectie in voorgevulde spuit
Noruega: Fyremadel 0,25 mg/0,5 ml injeksjonsvæske, oppløsning, i ferdigfylt sprøyte
Suecia: Fyremadel 0,25 mg/0,5 ml injektionsvätska, lösning, förfylld spruta
Reino Unido: Fyremadel 0.25 mg/0.5 ml solution for injection in pre-filled syringe
Fecha de la última revisión de este prospecto:Abril 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/ .
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FYREMADEL 0,25 MG / 0,5 ML SOLUCION INYECTABLE EN JERINGA PRECARGADA EFGForma farmacéutica: INYECTABLE, 0,25 mg/0,5 mlPrincipio activo: GanirelixFabricante: Gp Pharm S.A.Requiere recetaForma farmacéutica: INYECTABLE, 0,25 MG/0,5 MLPrincipio activo: GanirelixFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: GanirelixFabricante: Organon N.V.Requiere receta
Médicos online para FYREMADEL 0,25 MG / 0,5 ML SOLUCION INYECTABLE EN JERINGA PRECARGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FYREMADEL 0,25 MG / 0,5 ML SOLUCION INYECTABLE EN JERINGA PRECARGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






