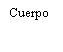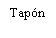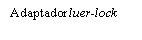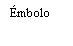FENDRIX, SUSPENSION INYECTABLE

Cómo usar FENDRIX, SUSPENSION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fendrix suspensión inyectable
Vacuna antihepatitis B (ADNr) (adyuvada, adsorbida)
Lea todo el prospecto detenidamente antes de recibir esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted, y no debe dársela a otras personas.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fendrix y para qué se utiliza
- Qué necesita saber antes de recibir Fendrix
- Cómo se administra Fendrix
- Posibles efectos adversos
- Conservación de Fendrix
- Contenido del envase e información adicional
1. Qué es Fendrix y para qué se utiliza
Fendrix es una vacuna que previene la hepatitis B.
Se utiliza en pacientes con problemas de riñón:
- pacientes sometidos a hemodiálisis, en los que una máquina de diálisis elimina los productos de desecho de la sangre
- pacientes que van a ser sometidos a hemodiálisis en el futuro.
Fendrix es para adultos y jóvenes a partir de los 15 años.
Qué es la hepatitis B
La hepatitis B está causada por un virus que hace que el hígado se hinche.
- Puede que no se observen los signos hasta haber transcurrido un periodo de 6 semanas a 6 meses después de la infección.
- Los principales signos de la enfermedad incluyen signos leves de una gripe, tales como dolor de cabeza o fiebre, sensación de cansancio extremo, orina de color oscuro, deposiciones (heces) de color pálido, ojos o piel de color amarillo (ictericia). Estos u otros signos pueden significar que la persona necesite tratamiento en el hospital. La mayoría de las personas se recuperan completamente de la enfermedad.
- Algunas personas con hepatitis B no parecen enfermas o no se sienten enfermas (no tienen ningún signo de la enfermedad).
- El virus se encuentra en fluidos corporales tales como en la vagina, sangre, semen o saliva (esputo).
Portadores de hepatitis B
- El virus de la hepatitis B permanece en el organismo de algunas personas durante toda su vida.
- Esto significa que pueden infectar a otras personas y se les conoce como portadores del virus.
- Es probable que los portadores del virus desarrollen problemas graves en el hígado, tales como cirrosis o cáncer de hígado.
Cómo actúa Fendrix
- Fendrix ayuda a que su organismo desarrolle su propia protección frente al virus (anticuerpos). Estos anticuerpos son los que le protegerán frente a la enfermedad.
- Fendrix contiene dos sustancias denominadas MPL (un derivado graso purificado no tóxico de origen bacteriano) y fosfato de aluminio. Ambas permiten que la vacuna funcione más rápido, mejor y durante más tiempo.
- Como con todas las vacunas, un ciclo de vacunación con Fendrix puede no proteger completamente a todas las personas vacunadas.
- Puede que Fendrix no le proteja de la enfermedad si usted ya ha sido infectado por el virus de la hepatitis B.
- Fendrix sólo puede protegerle frente a la infección por el virus de la hepatitis B. No puede protegerle frente a otras infecciones que puedan afectar al hígado, incluso si estas infecciones tienen signos similares a los causados por el virus de la hepatitis B.
2. Qué necesita saber antes de recibir Fendrix
No se debe administrar Fendrix
- si es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Los signos de una reacción alérgica pueden incluir: erupción de la piel con picor, dificultad para respirar e hinchazón de la cara o la lengua
- si ha tenido alguna vez una reacción alérgica a cualquier vacuna frente a la hepatitis B
- si tiene una infección grave con fiebre. Se puede administrar la vacuna una vez que se haya recuperado. Una infección de poca importancia, como un resfriado, no debería ser un problema para la vacunación, pero dígaselo primero a su médico.
No se debe administrar Fendrix si alguna de las situaciones anteriores le afecta. Si no está seguro, consulte a su médico o farmacéutico antes de recibir Fendrix.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de que se le administre Fendrix:
- si tiene alergias conocidas
- si ha tenido algún problema de salud después de la administración de una vacuna con anterioridad.
Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermero si usted se ha desmayado en anteriores ocasiones tras la administración de una inyección.
Si alguna de las situaciones anteriores le afecta (o no está seguro), consulte con su médico o farmacéutico antes de recibir Fendrix.
Otros medicamentosy Fendrix
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento o si ha recibido recientemente cualquier otra vacuna.
- Debería disponer de un margen de, al menos, 2 a 3 semanas entre la administración de Fendrix y cualquier otra vacuna.
- Puede que Fendrix tenga que administrarse al mismo tiempo que una inyección de inmunoglobulinas de hepatitis B. Su médico se asegurará de que las vacunas se le administran en diferentes partes del cuerpo.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de que se le administre esta vacuna.
Conducción y uso de máquinas
Puede sentirse cansado o tener dolor de cabeza después de recibir Fendrix. Si le ocurre esto, tenga especial cuidado mientras conduzca o maneje herramientas o máquinas.
Fendrix contiene sodio
Esta vacuna contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Fendrix
Cómo se administra la vacuna
El médico o el enfermero le inyectará Fendrix en un músculo, normalmente en la parte superior de su brazo.
Cuánto se administra
- Usted recibirá una serie de cuatro inyecciones.
- Se le administrarán las inyecciones en un periodo de 6 meses:
- Primera inyección: en la fecha acordada con su médico.
- Segunda inyección: 1 mes después de la primera inyección
- Tercera inyección: 2 meses después de la primera inyección.
- Cuarta inyección: 6 meses después de la primera inyección.
- El médico o el enfermero le dirá cuando debe volver para las siguientes inyecciones.
- Una vez que haya recibido la primera inyección de Fendrix, es necesario que las siguientes inyecciones sean también de Fendrix (no otro tipo de vacuna para la hepatitis B).
Su médico le dirá si necesita alguna inyección adicional o de recuerdo en el futuro. Fendrix puede utilizarse también como dosis de recuerdo después de un ciclo de vacunación con un tipo diferente de vacuna para la hepatitis B.
Si no recibe una dosis
- Si usted no recibe una inyección, consulte a su médico y concierte otra cita.
- Asegúrese de que termina el ciclo de vacunación completo de cuatro inyecciones. En caso contrario, puede no estar completamente protegido frente a la enfermedad.
4. Posibles efectos adversos
Al igual que todos los medicamentos, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes efectos adversos pueden ocurrir con esta vacuna. Su frecuencia se determina utilizando la siguiente definición:
Muy frecuentes(estos pueden ocurrir con más de 1 de cada 10 dosis de la vacuna): dolor de cabeza, sensación de cansancio, dolor o molestias en donde se administró la inyección.
Frecuentes(estos pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna): enrojecimiento o hinchazón en donde se administró la inyección, fiebre, problemas de digestión y de estómago.
Poco frecuentes(estos pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): escalofríos, erupción con enrojecimiento de la piel, otras reacciones en donde se administró la inyección.
Raros(estos pueden ocurrir hasta con 1 de cada 1.000 dosis de la vacuna): reacciones alérgicas, sofocos, sensación de mareo, sensación de sed, sensación de nerviosismo, infección causada por un virus, dolor de espalda, inflamación de los tendones.
Además, se han notificado los siguientes efectos adversos con otras vacunas frente a la hepatitis B:
Muy raros(estos pueden ocurrir hasta con 1 de cada 10.000 dosis de la vacuna): ataques, desmayos, problemas con los nervios de su ojo (neuritis óptica), esclerosis múltiple, pérdida de sensibilidad o problemas al mover algunas partes de su cuerpo, dolor de cabeza grave con rigidez de cuello, entumecimiento o debilidad de brazos y piernas (neuropatía), inflamación de los nervios (neuritis), debilidad y parálisis de las extremidades progresando con frecuencia al pecho y a la cara (síndrome de Guillain-Barré), hinchazón o infección del cerebro (encefalitis, encefalopatía).
Reacciones alérgicas, incluyendo reacciones anafilactoides, también pueden ocurrir muy raramente (hasta con 1 de cada 10.000 dosis de la vacuna). Pueden ser erupciones cutáneas locales o diseminadas con picor o con ampollas, hinchazón de los ojos y la cara, dificultad para respirar o tragar, bajada repentina de la presión sanguínea y pérdida de consciencia. Estas reacciones pueden ocurrir antes de que usted abandone la consulta del médico. No obstante, busque inmediatamente tratamiento médico en cualquier caso.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fendrix
- Mantener esta vacuna fuera de la vista y del alcance de los niños.
- No utilice esta vacuna después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC).
- Conservar en el embalaje original para protegerlo de la luz.
- No congelar. La congelación destruye la vacuna.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fendrix
- El principio activo en 1 dosis (0,5 ml) de Fendrix es:
Antígeno de superficie del virus de la Hepatitis B 1, 2, 3 20 microgramos
1adyuvado por AS04C que contiene:
3-O-desacil-4’- monofosforil lípido A (MPL) 2 50 microgramos
2adsorbido en fosfato de aluminio (0,5 miligramos de Al3+ en total)
3producido por la tecnología del ADN recombinante en células de levadura (Saccharomyces cerevisiae)
- Los demás componentes de Fendrix son: cloruro de sodio, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Fendrix es una suspensión blanca y lechosa.
Fendrix está disponible en jeringa precargada de 1 dosis con o sin agujas separadas, tamaños de envase de 1 y 10.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación:
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 | Lietuva GlaxoSmithKline Biologicals SA Tel. +370 80000334 |
???????? GlaxoSmithKline Biologicals SA ???. + 359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Magyarország GlaxoSmithKline Biologicals SA Tel.: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: + 356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)30 69 38 100 |
Eesti GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλ?δα GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Tηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 970 75-0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 Hrvatska GlaxoSmithKline Biologicals SA Tel.: + 385 800787089 | Portugal Smith Kline & French Portuguesa - Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 România GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenija GlaxoSmithKline Biologicals SA Tel: + 386 80688869 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Biologicals SA Tel: + 421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel:+ 39 (0)45 774 1111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κ?προς GlaxoSmithKline Biologicals SA Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Biologicals SA Tel: + 371 80205045 | United Kingdom (Northern Ireland) GlaxoSmithKline Biologicals SA Tel: +44 (0)800 221 441 |
Fecha de la última revisión de este prospecto:04/2023
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu, y en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
--------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
Durante la conservación de la vacuna, se puede observar un fino depósito blanco y un sobrenadante transparente.
Antes de la administración, la vacuna se debe agitar bien, para obtener una suspensión blanca ligeramente opaca.
La vacuna se debe examinar visualmente antes y después de la resuspensión para observar si existe alguna partícula extraña y/o cambio del aspecto físico. La vacuna no se debe utilizar si ha tenido lugar algún cambio en el aspecto de la vacuna.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
Fendrix no se debe administrar a sujetos con hipersensibilidad al principio activo o a cualquiera de los excipientes.
Fendrix no se debe administrar a sujetos con hipersensibilidad después de una administración anterior de otras vacunas de hepatitis B.
Fendrix no se debe administrar a sujetos que padecen enfermedades febriles agudas graves. La presencia de una infección de poca importancia, como un catarro, no es una contraindicación para la vacunación.
Fendrix se debe inyectar por vía intramuscular en la región deltoidea.
Se debe evitar la administración intramuscular en el músculo glúteo ya que puede conducir a una respuesta inmunitaria subóptima a la vacuna.
Bajo ninguna circunstancia se debe administrar Fendrix por vía intradérmica o intravenosa.
Debido a que los pacientes pre-hemodializados y hemodializados están particularmente expuestos al VHB y tienen un mayor riesgo de estar infectados de forma crónica, se debe considerar una actitud preventiva, es decir, se administrará una dosis de recuerdo para asegurar un nivel protector de anticuerpos en base a lo definido por las recomendaciones y directrices locales.
Se deberá disponer en todo momento del tratamiento médico adecuado, para el caso poco común de que se presente una reacción anafiláctica tras la administración de la vacuna.
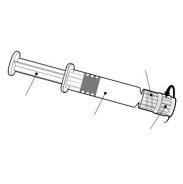
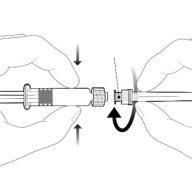
Instrucciones para la jeringa precargada
| Sostenga la jeringa por el cuerpo, no por el émbolo. Desenrosque el tapón de la jeringa girándola en sentido contrario a las agujas del reloj. |
| Para insertar la aguja, conecte la base al adaptador luer-locky gírelo un cuarto de vuelta en el sentido de las agujas del reloj hasta que sienta que se bloquea. No saque el émbolo de la jeringa del cuerpo. Si esto ocurre, no administre la vacuna. |
Eliminación de residuos
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FENDRIX, SUSPENSION INYECTABLEForma farmacéutica: INYECTABLE, 20 mcg ANTIGENO SUPERFICIE HEPATITIS B/ mlPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INYECTABLE, 10 mcg ANTIGENO SUPERFICIE HEPATITIS B/ 0,5 mlPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INYECTABLE, 20 µgPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline BiologicalsRequiere receta
Médicos online para FENDRIX, SUSPENSION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FENDRIX, SUSPENSION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes