
ESCITALOPRAM SANDOZ 15 MG COMPRIMIDOS BUCODISPERSABLES EFG

Cómo usar ESCITALOPRAM SANDOZ 15 MG COMPRIMIDOS BUCODISPERSABLES EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Escitalopram Sandoz 10 mg comprimidos bucodispersables EFG
Escitalopram Sandoz 15mg comprimidos bucodispersables EFG
Escitalopram Sandoz 20 mg comprimidos bucodispersables EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Escitalopram Sandoz y para qué se utiliza.
- Qué necesita saber antes de empezar a tomar Escitalopram Sandoz.
- Cómo tomar Escitalopram Sandoz.
- Posibles efectos adversos.
- Conservación de Escitalopram Sandoz.
- Contenido del envase e información adicional.
1. Qué es Escitalopram Sandoz y para qué se utiliza
Escitalopram Sandoz contiene el principio activo escitalopram. Escitalopram pertenece a un grupo de antidepresivos denominados inhibidores selectivos de la recaptación de serotonina (ISRSs). Estos medicamentos actúan sobre el sistema serotoninérgico en el cerebro aumentando el nivel de serotonina. Las alteraciones del sistema serotoninérgico se consideran un factor importante en el desarrollo de la depresión y enfermedades relacionadas.
Escitalopram Sandoz contiene escitalopram y está indicado para el tratamiento de la depresión (episodios depresivos mayores) y trastornos de ansiedad (tales como trastorno de angustia con o sin agorafobia, trastorno de ansiedad social, trastorno de ansiedad generalizada y trastorno obsesivo-compulsivo).
Pueden pasar un par de semanas antes de que empiece a sentirse mejor. Continúe tomando escitalopram aunque tarde un tiempo en notar alguna mejoría.
Debe consultar a un médico si empeora o si no mejora.
2. Qué necesita saber antes de empezar a tomar Escitalopram Sandoz
No tome Escitalopram Sandoz
- si es alérgico a escitalopram o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si toma otros medicamentos que pertenecen al grupo denominado inhibidores de la MAO, incluyendo selegilina (utilizada para el tratamiento de la enfermedad de Parkinson), moclobemida (utilizada para el tratamiento de la depresión) y linezolid (un antibiótico),
- si padece de nacimiento o ha sufrido un episodio de alteración de la frecuencia cardiaca (detectado en un ECG, prueba que evalúa el funcionamiento del corazón),
- si está tomando medicamentos para problemas de ritmo cardiaco o que puedan afectar el ritmo cardiaco (ver sección 2 “Otros medicamentos y Escitalopram Sandoz”).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Escitalopram Sandoz. Informe a su médico si padece algún otro trastorno o enfermedad, ya que su médico puede necesitar tenerlo en cuenta. En concreto, informe a su médico:
- si padece epilepsia. El tratamiento con Escitalopram Sandoz se debe interrumpir si se producen convulsiones por primera vez, o si observa un incremento en la frecuencia de las convulsiones (ver también sección 4 “Posibles efectos adversos”),
- si tiene alteraciones en la función del hígado o riñones. Puede que su médico necesite ajustar su dosis,
- si padece diabetes. El tratamiento con Escitalopram Sandoz puede alterar el control glucémico. Puede ser necesario un ajuste de la dosis de insulina y/o hipoglucemiante oral,
- si tiene disminución de los niveles de sodio en sangre,
- si tiende a desarrollar fácilmente hemorragias o cardenales, o si está embaraza (ver “Embarazo, lactancia y fertilidad),
- si está recibiendo tratamiento electroconvulsivo,
- si padece una enfermedad coronaria,
- si padece o ha padecido problemas de corazón o ha sufrido recientemente un ataque al corazón,
- si el ritmo de su corazón en reposo es lento y/o sabe que puede tener una disminución de sal como resultado de una diarrea grave y prolongada y vómitos (estando enfermo) o uso de diuréticos,
- si experimenta latidos del corazón rápidos o irregulares, desfallecimiento, colapso o mareo al levantarse, que puede indicar un funcionamiento anómalo del ritmo del corazón,
- si tiene o ha tenido anteriormente problemas de ojos, como ciertos tipos de glaucoma (incremento de la tensión en el ojo).
Tenga en cuenta
Algunos pacientes con enfermedad maníaco-depresiva pueden entrar en una fase maníaca. Esto se caracteriza por un cambio de ideas poco común y rápido, alegría desproporcionada y una actividad física excesiva. Si usted lo experimenta, contacte con su médico.
Síntomas como inquietud o dificultad para sentarse o estar de pie, pueden ocurrir también durante las primeras semanas de tratamiento. Informe a su médico inmediatamente si experimenta estos síntomas.
Algunos medicamentos como escitalopram (también llamados ISRS/IRSN) pueden causar síntomas de disfunción sexual (ver sección 4). En algunos casos, estos síntomas persisten después de suspender el tratamiento.
Pensamientos suicidas y empeoramiento de la depresión o trastorno de ansiedad
Si se encuentra deprimido y/o sufre un trastorno de ansiedad, puede que en algunas ocasiones tenga pensamientos de dañarse a sí mismo o de suicidio. Éstos pueden ir aumentando al tomar antidepresivos por primera vez, puesto que todos estos medicamentos requieren un tiempo para empezar a hacer efecto, generalmente alrededor de unas dos semanas, aunque en algunos casos puede ser mayor el tiempo.
Puede ser más propenso a tener este tipo de pensamientos:
- si previamente ha tenido pensamientos de suicidio o autolesión,
- si es un adulto joven. Información de ensayos clínicos ha demostrado un aumento del riesgo de conductas suicidas en adultos menores de 25 años con enfermedades psiquiátricas que fueron tratados con un antidepresivo.
Si en cualquier momento tiene pensamientos de autolesionarse o de suicidio, contacte con su médico o diríjase directamente a un hospital.
Puede ayudarle decir a un pariente o un amigo cercanoque está deprimido o que tiene un trastorno de ansiedad y pedirle que lea este prospecto. Puede preguntarles si piensan que su depresión o trastorno de ansiedad ha empeorado, o si están preocupados por los cambios en su actitud.
Niños y adolescentes
Escitalopram Sandoz no se debe utilizar normalmente en el tratamiento de niños y adolescentes menores de 18 años. Asimismo, debe saber que en pacientes menores de 18 años existe un mayor riesgo de efectos adversos como intentos de suicidio, pensamientos de suicidio y hostilidad (predominantemente agresión, comportamiento de confrontación e irritación) cuando ingieren esta clase de medicamentos. Pese a ello, su médico puede prescribir Escitalopram Sandoz comprimidos bucodispersables a pacientes menores de 18 años cuando decida qué es lo más conveniente para el paciente. Si su médico ha prescrito Escitalopram Sandoz comprimidos bucodispersables a un paciente menor de 18 años y desea discutir esta decisión, por favor, vuelva a su médico. Debe informar a su médico si alguno de los síntomas descritos anteriormente progresa o experimenta complicaciones cuando pacientes menores de 18 años están tomando Escitalopram Sandoz comprimidos bucodispersables. Además, los efectos a largo plazo en lo que concierne a seguridad, en el crecimiento, madurez y el desarrollo cognitivo y conductual de Escitalopram Sandoz comprimidos bucodispersables en este grupo de edad no han quedado demostrados todavía.
Otros medicamentos y Escitalopram Sandoz
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Informe a su médico si está tomando alguno de los siguientes medicamentos:
- “Inhibidores no selectivos de la monoaminooxidasa (IMAOs)”, que contengan fenelzina, iproniazida, isocarboxazida, nialamida y tranilcipromina como principios activos. Si ha tomado alguno de estos medicamentos tiene que esperar 14 días antes de empezar a tomar Escitalopram Sandoz. Después de terminar con Escitalopram Sandoz deben transcurrir 7 días antes de tomar alguno de estos medicamentos.
- “Inhibidores selectivos de la MAO-A reversibles”, que contengan moclobemida (para el tratamiento de la depresión).
- “Inhibidores de la MAO-B irreversibles”, que contengan selegilina (para el tratamiento de la enfermedad de Parkinson). Estos incrementan el riesgo de efectos secundarios.
- El antibiótico linezolid.
- Litio (para el tratamiento del trastorno maníaco-depresivo) y triptófano.
- Imipramina y desipramina (ambos para el tratamiento de la depresión).
- Sumatriptán y medicamentos similares (para el tratamiento de la migraña) y tramadol (utilizado contra el dolor intenso). Éstos aumentan el riesgo de efectos secundarios.
- Buprenorfina(utilizado para el dolor intenso) ya que incrementa el riesgo de síndorme serotoninérgico, un sindrome potencialmente mortal.
- Cimetidina, lansoprazol y omeprazol (para el tratamiento de las úlceras de estómago), fluconazol (para el tratamiento de infecciones por hongos), fluvoxamina (antidepresivo) y ticlopidina (para reducir el riesgo de accidente cerebro-vascular). Estos pueden causar aumento de las concentraciones en sangre de escitalopram.
- Hierba de San Juan (Hypericum perforatum) – planta medicinal utilizada para la depresión.
- Ácido acetilsalicílico y medicamentos antiinflamatorios no esteroideos (medicamentos utilizados para aliviar el dolor o para reducir el riesgo de trombosis, también llamados anticoagulantes). Éstos pueden aumentar la tendencia a hemorragias.
- Warfarina, dipiridamol y fenprocumón (medicamentos utilizados para fluidificar la sangre, también llamados anticoagulantes). Su médico controlará probablemente el tiempo de coagulación de la sangre al inicio y al final del tratamiento con Escitalopram Sandoz, para comprobar que la dosis de anticoagulante es todavía adecuada.
- Mefloquina (para el tratamiento de la malaria), bupropion (para el tratamiento de la depresión) y tramadol (para el tratamiento del dolor intenso) debido al posible riesgo de disminuir el umbral de convulsiones.
- Neurolépticos (medicamentos para el tratamiento de la esquizofrenia, psicosis) y antidepresivos (antidepresivos tricíclicos e ISRSs) debido al posible riesgo de disminuir el umbral de convulsiones.
- Flecainida, propafenona y metoprolol (usados en enfermedades cardiovasculares), clomipramina y nortriptilina (antidepresivos) y risperidona, tioridazina y haloperidol (antipsicóticos). Puede ser que la dosis de Escitalopram Sandoz necesite ser ajustada.
- Medicamentos que disminuyen los niveles de potasio o magnesio en sangre ya que ello incrementa el riesgo de sufrir alteraciones del ritmo del corazón, que suponen un riesgo para la vida.
No tome Escitalopram Sandoz si está tomando medicamentos para problemas del ritmo cardiaco o que puedan afectar el ritmo cardiaco, p. ej., antiarrítmicos Clase IA y III, antipsicóticos (p. ej., derivados de fenotiazina, pimozida, haloperidol), antidepresivos tricíclicos, algunos antimicrobianos (p. ej., esparfloxacino, moxifloxacino, eritromicina IV, pentamidina, tratamiento antimalaria particularmente halofantrina), algunos antihistamínicos (astemizol, hidroxicina, mizolastina). Contacte con su médico para cualquier consulta adicional.
Toma de Escitalopram Sandoz con alimentos, bebidas y alcohol
Escitalopram Sandoz comprimidos bucodispersables se puede tomar con o sin alimentos (ver sección 3 “Cómo tomar Escitalopram Sandoz”).
Como con muchos medicamentos, no se recomienda la combinación de Escitalopram Sandoz y alcohol, aunque no se espera que Escitalopram Sandoz interaccione con alcohol.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No tome escitalopram si está embarazada o en periodo de lactancia a menos que usted y su médico hayan analizado los riesgos y beneficios implicados.
Si toma escitalopram durante los últimos 3 meses del embarazo tiene que ser consciente que se pueden observar en el bebé recién nacido los siguientes efectos: dificultad respiratoria, piel azulada, ataques, cambios de la temperatura corporal, dificultades para alimentarse, vómitos, azúcar bajo en sangre, rigidez o flojedad muscular, reflejos intensos, temblores, inquietud, irritabilidad, letargo, lloro constante, somnolencia y dificultades para dormirse. Si su bebé recién nacido tiene alguno de estos síntomas, contacte con su médico inmediatamente.
Asegúrese que su comadrona y/o médico saben que está en tratamiento con escitalopram.
Durante el embarazo, particularmente en los últimos 3 meses, medicamentos como escitalopram pueden aumentar el riesgo de una enfermedad grave en recién nacidos, denominada hipertensión pulmonar persistente neonatal (HPPN), en la cual el bebé respira rápido y se pone azulado. Estos síntomas suelen empezar durante las primeras 24 h después del nacimiento. Si aparecen en su bebé debe contactar con su comadrona y/o médico inmediatamente.
Si escitalopram se utiliza durante el embarazo, nunca se debe interrumpir bruscamente.
Si toma escitalopram en la etapa final del embarazo se puede producir un mayor riesgo de sangrado vaginal abundante poco después del parto, especialmente si tiene antecedentes de alteraciones hemorrágicas. Su médico o matrona deben saber que usted está tomando escitalopram para poderle aconsejar.
Es de esperar que escitalopram se excrete a través de la leche materna.
Citalopram, un medicamento similar a escitalopram, ha demostrado reducir la calidad del esperma en modelos animales. Teóricamente, este efecto podría afectar la fertilidad, pero hasta la fecha no se ha observado su impacto en la fertilidad humana.
Conducción y uso de máquinas
No conduzca ni utilice herramientas o máquinas hasta que sepa cómo le afecta el tratamiento con escitalopram.
Escitalopram Sandoz contiene lactosay sodio
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por comprimido bucodispersable; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Escitalopram Sandoz
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Los comprimidos bucodispersables de Escitalopram Sandoz se toman todos los días como una sola dosis diaria. Puede tomar Escitalopram Sandoz con o sin alimentos.
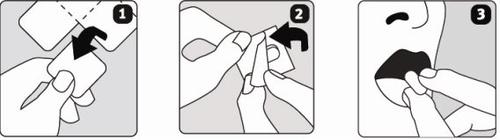
Los comprimidos bucodispersables de Escitalopram Sandoz se rompen con facilidad, por lo que debe manipular los comprimidos con cuidado. No manipule los comprimidos con las manos húmedas ya que los comprimidos se pueden romper.
- Sujete la tira de blíster por los bordes y separe un blíster del resto de la tira rasgando suavemente a lo largo de la línea perforada.
- Retire cuidadosamente la solapa trasera del blíster.
- Ponga el comprimido en la lengua. El comprimido se desintegrará rápidamente y puede ser tragado sin agua.
Escitalopram Sandoz comprimidos bucodispersables no está disponible para todas las dosis que se describen a continuación. Para estas dosis, debe tomar otros medicamentos disponibles en el mercado. Consulte a su médico o farmacéutico.
Adultos
Depresión
La dosis recomendada de Escitalopram Sandoz es de 10 mg al día tomados como dosis única. Su médico puede aumentarla hasta un máximo de 20 mg al día.
Trastorno de angustia
La dosis inicial de Escitalopram Sandoz es de 5 mg al día como dosis única durante la primera semana antes de aumentar la dosis a 10 mg al día. Su médico puede aumentarla posteriormente hasta un máximo de 20 mg al día.
Trastorno de ansiedad social
La dosis recomendada de Escitalopram Sandoz es de 10 mg al día tomados como dosis única. Su médico puede disminuir su dosis a 5 mg al día o aumentar la dosis hasta un máximo de 20 mg al día, dependiendo de cómo responda al medicamento.
Trastorno de ansiedad generalizada
La dosis recomendada de Escitalopram Sandoz es de 10 mg al día tomados como dosis única. La dosis puede ser aumentada por su médico hasta un máximo de 20 mg al día.
Trastorno obsesivo-compulsivo
La dosis recomendada de Escitalopram Sandoz es de 10 mg tomados al día como dosis única. La dosis puede ser aumentada por su médico hasta un máximo de 20 mg al día.
Pacientes de edad avanzada (mayores de 65 años)
La dosis inicial recomendada de Escitalopram Sandoz es de 5 mg al dia tomados como dosis única. La dosis puede ser aumentada por su médico hasta 10 mg al día.
Uso en niños y adolescentes
Escitalopram Sandoz normalmente no se debe administrar a niños y adolescentes. Para información adicional ver sección 2 “Qué necesita saber antes de empezar a tomar Escitalopram Sandoz”.
Insuficiencia renal
Se aconseja precaución en pacientes con función renal gravemente disminuida. Tómelo según le prescriba su médico.
Insuficiencia hepática
Los pacientes con problemas hepáticos no deben tomar más de 10 mg al día. Tómelo según le prescriba su médico.
Pacientes considerados como metabolizadores lentos de la CYP2C19
Los pacientes con este genotipo conocido no deben tomar más de 10 mg al día. Tómelo según le prescriba su médico.
Duración del tratamiento
Pueden pasar un par de semanas antes de que se empiece a sentir mejor. Siga tomando escitalopram incluso si se empieza a sentir mejor antes de lo previsto en su condición.
No modifique la dosis del medicamento sin hablar antes con su médico.
Siga tomando escitalopram el tiempo recomendado por su médico. Si interrumpe el tratamiento demasiado pronto, los síntomas pueden reaparecer. Se recomienda que el tratamiento continúe durante como mínimo 6 meses después de volver a encontrarse bien.
Si toma más Escitalopram Sandoz del que debe
Si toma más de la dosis prescrita de Escitalopram Sandoz, consulte inmediatamente a su médico, vaya al servicio de urgencias del hospital más cercano o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida. Hágalo incluso cuando no observe molestias o signos de intoxicación. Algunos de los signos de sobredosis pueden ser mareos, temblor, agitación, convulsión, coma, náuseas, vómitos, cambios en el ritmo cardiaco, disminución de la tensión sanguínea y cambios en el equilibrio hidro/salino corporal. Lleve el envase de Escitalopram Sandoz si acude al médico o al hospital.
Si olvidó tomar Escitalopram Sandoz
No tome una dosis doble para compensar las dosis olvidadas. Si olvidó tomar una dosis, y lo recuerda antes de irse a la cama, tómela enseguida. Al día siguiente siga como siempre. Si se acuerda durante la noche o al día siguiente, deje la dosis olvidada y siga como siempre.
Si interrumpe el tratamiento con Escitalopram Sandoz
No interrumpa el tratamiento con Escitalopram Sandoz hasta que su médico se lo diga. Cuando haya terminado su curso de tratamiento, generalmente se recomienda que la dosis de Escitalopram Sandoz sea reducida gradualmente durante varias semanas.
Cuando deja de tomar Escitalopram Sandoz, especialmente si es de forma brusca, puede sentir síntomas de retirada. Éstos son frecuentes cuando se suspende el tratamiento con escitalopram. El riesgo es mayor cuando escitalopram se ha utilizado durante largo tiempo, en elevadas dosis o cuando la dosis se reduce demasiado rápido. La mayoría de las personas encuentran que estos síntomas son leves y desaparecen por si mismos en dos semanas. Sin embargo, en algunos pacientes pueden ser intensos o prolongados (2 a 3 meses o más). Si tiene síntomas graves de retirada cuando deja de tomar escitalopram, contacte con su médico. Puede pedirle que vuelva a tomar los comprimidos de nuevo y los deje más lentamente.
Los síntomas de retirada incluyen: sensación de vértigo (inestable o sin equilibrio), sensación de hormigueo, sensación de escozor y (con menos frecuencia) de shock eléctrico, incluso en la cabeza, alteraciones del sueño (sueños demasiado intensos, pesadillas, incapacidad de dormir), sensación de intranquilidad, dolor de cabeza, sensación de mareo (náuseas), sudoración (incluidos sudores nocturnos), sensación de inquietud o agitación, temblor (inestabilidad), sentimiento de confusión o desorientación, sentimientos de emoción o irritación, diarrea (heces sueltas), alteraciones visuales, pulsación rápida o palpitaciones.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos normalmente desaparecen después de pocas semanas de tratamiento. Sea consciente de que muchos de los efectos pueden ser síntomas de su enfermedad y por tanto mejoraran cuando se empiece a encontrar mejor.
Si tiene alguno de los siguientes síntomas debe contactar con su médico o ir al hospital de inmediato:
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- sangrados inusuales, incluyendo sangrados gastrointestinales.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- hinchazón de la piel, lengua, labios o cara, o tiene dificultades respiratorias o de deglución (reacción alérgica),
- fiebre elevada, agitación, confusión, temblores y contracciones repentinas de músculos, pueden ser signos de una situación poco común denominada síndrome serotoninérgico (ver sección 2).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- dificultades para orinar,
- convulsiones (ataques), ver también la sección “Advertencias y precauciones”,
- piel amarillenta y blanqueamiento en los ojos, son signos de alteración de la función hepática /hepatitis,
- si experimenta latidos del corazón rápidos o irregulares o desfallecimiento, síntomas que pueden indicar una condición de riesgo para la vida conocida como Torsade de Pointes,
- pensamientos de dañarse a sí mismo (autolesión) o pensamientos de suicidio, ver también la sección “Advertencias y precauciones”.
Además de lo indicado anteriormente, se han notificado los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- sentirse mareado (náuseas),
- dolor de cabeza.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- taponamiento o mucosidad nasal (sinusitis),
- disminución o incremento del apetito,
- ansiedad, agitación, sueños extraños, dificultad para conciliar el sueño, sentirse dormido, mareos, bostezos, temblores, picores en la piel,
- diarrea, estreñimiento, vómitos, sequedad de boca,
- aumento de la sudoración,
- dolores musculares y articulares (artralgia y mialgia),
- alteraciones sexuales (retraso de la eyaculación, problemas con la erección, disminución de la conducta sexual y las mujeres pueden experimentar dificultades para alcanzar el orgasmo),
- fatiga, fiebre,
- aumento de peso.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- urticaria, erupción cutánea, picores (prurito),
- rechinar de dientes, agitación, nerviosismo, crisis de angustia, confusión,
- alteraciones del sueño, alteraciones del gusto, desmayos (síncope),
- dilatación de pupilas (midriasis), alteración visual, zumbidos en los oídos (tinnitus),
- pérdida de pelo,
- hemorragia menstrual excesiva,
- periodo menstrual irregular,
- disminución de peso,
- ritmo del corazón rápido,
- hinchazón de brazos y piernas,
- hemorragia nasal.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- agresión, despersonalización, alucinaciones,
- ritmo del corazón bajo.
Frecuencia no conocida(no puede estimarse partir de los datos disponibles):
- disminución de los niveles de sodio en la sangre (los síntomas son sentirse mareado y malestar con debilidad muscular o confusión),
- mareos al ponerse de pie debido a la tensión sanguínea baja (hipotensión ortostática),
- pruebas de la función hepática alteradas (aumento de las enzimas hepáticas en la sangre),
- trastornos del movimiento (movimientos involuntarios de los músculos),
- erecciones dolorosas (priapismo),
- signos de aumento del sangrado p. ej., de la piel o mucosas (equimosis),
- hinchazón repentina de piel o mucosas (angioedema),
- incremento de la cantidad de orina excretada (secreción inadecuada de la hormona ADH),
- producción de leche en hombres y en mujeres que no están en período de lactancia,
- manía,
- se ha observado un aumento del riesgo de fracturas óseas en pacientes tratados con este tipo de medicamentos,
- alteración del ritmo del corazón (denominada “prolongación del intervalo QT”, observada en el ECG, actividad eléctrica del corazón),
- sangrado vaginal abundante poco después del parto (hemorragia posparto), ver «Embarazo, lactancia y fertilidad» en la sección 2 para más información.
Se conocen otros efectos adversos que aparecen con medicamentos que actúan de forma parecida a escitalopram (principio activo de Escitalopram Sandoz), estos son:
- inquietud motora (acatisia),
- pérdida de apetito.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Escitalopram Sandoz
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y blíster después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación.
Conservar en el embalaje original para protegerlo de la luz y humedad.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Escitalopram Sandoz
- El principio activo es escitalopram.
Escitalopram Sandoz 10 mg: cada comprimidos bucodispersables contiene 10 mg de escitalopram (como oxalato).
Escitalopram Sandoz 15 mg: cada comprimidos bucodispersables contiene15 mg de escitalopram (como oxalato).
Escitalopram Sandoz 20 mg: cada comprimidos bucodispersables contiene 20 mg de escitalopram (como oxalato).
- Los demás componentes son: celulosa microcristalina, lactosa monohidrato, croscarmelosa sódica, polacrilina potásica, acesulfamo potásico, neohesperidina dihidrochalcona, estearato de magnesio, aroma de menta [contiene: maltodextrina (de maíz), almidón modificado E 1450 (almidón de maíz ceroso) y aceite de menta (menta arvensis)], ácido clorhídrico concentrado (para ajuste de pH).
Aspecto del producto y contenido del envase
Escitalopram Sandoz 10 mg comprimidos bucodispersables: comprimidos de color blanco a blanco opaco, redondos, planos con bordes biselados, un diámetro de 9 mm y grabado con "10" en un lado.
Escitalopram Sandoz 15 mg comprimidos bucodispersables: comprimidos de color blanco a blanco opaco, redondos, planos con bordes biselados, un diámetro de 11 mm y grabado con "15" en un lado.
Escitalopram Sandoz 20 mg comprimidos bucodispersables: comprimidos de color blanco a blanco opaco, redondos, planos con bordes biselados, un diámetro de 12 mm y grabado con "20" en un lado.
Escitalopram Sandoz comprimidos bucodispersables se envasa en blísteres de papel pelable / PET / aluminio // PVC / aluminio / oPA, insertados en un envase de cartón.
Tamaños de envase:
7, 10, 12, 14, 20, 28, 30, 35, 50, 56, 60, 90, 98, 100 y 200 comprimidos bucodispersables.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
España
Responsable de la fabricación
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1,
39179 Barleben
Alemania
o
Genepharm S.A.
18th km Marathon Avenue,
Pallini Attiki, 15351
Grecia
o
LEK, S.A.
Ul Domaniewska 50 C
Varsovia, PL 02-672
Polonia
o
Lek Pharmaceuticals d.d.
Verovškova, 57
SL-1526 Ljubljana
Eslovenia
o
S.C. Sandoz, S.R.L.
7A Livezeni Street, Targu-Mures
540472 Mures County, Rumanía
o
Rontis Hellas S.A. Medical and Pharmaceutical Products
Industrial Area of Larissa, P.O. Box 3012
GR41004 Larissa, Grecia
o
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park,
Paola, PLA 3000
Malta
Fecha de la última revisión de este prospecto:11/2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia13.11 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ESCITALOPRAM SANDOZ 15 MG COMPRIMIDOS BUCODISPERSABLES EFGForma farmacéutica: COMPRIMIDO, 10 mg escitalopramPrincipio activo: escitalopramFabricante: Lundbeck Espana S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 15 mg escitalopramPrincipio activo: escitalopramFabricante: Lundbeck Espana S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 20 mg escitalopramPrincipio activo: escitalopramFabricante: Lundbeck Espana S.A.Requiere receta
Médicos online para ESCITALOPRAM SANDOZ 15 MG COMPRIMIDOS BUCODISPERSABLES EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ESCITALOPRAM SANDOZ 15 MG COMPRIMIDOS BUCODISPERSABLES EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











