
EDUNIX 32 MG COMPRIMIDOS DE LIBERACION PROLONGADA

Cómo usar EDUNIX 32 MG COMPRIMIDOS DE LIBERACION PROLONGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Edunix 32 mg comprimidos de liberación prolongada
Hidrocloruro de Hidromorfona
Para uso en adultos y adolescentes a partir de 12 años
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Edunix y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Edunix
- Cómo tomar Edunix
- Posibles efectos adversos
- Conservación de Edunix
- Contenido del envase e información adicional
1. Qué es Edunix y para qué se utiliza
Edunix es un analgésico fuerte del grupo de medicamentos llamados analgésicos opiáceos.
Los comprimidos están indicados para tratar el dolor intenso.
2. Qué necesita saber antes de empezar a tomar Edunix
No tome Edunix:
- si es alérgico al hidrocloruro de hidromorfona o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si tiene problemas respiratorios (depresión respiratoria o trastorno pulmonar obstructivo crónico grave);
- si tiene un trastorno grave y persistente de las vías aéreas con estrechamiento de las vías respiratorias (por ejemplo asma grave);
- en caso de pérdida de conocimiento (coma);
- si tiene problemas de estómago o dolor abdominal inesperado (abdomen agudo);
- si usted tiene un problema intestinal con ausencia de motilidad (íleo paralítico);
- si está tomando inhibidores de la monoamino oxidasa (IMAOs - medicamentos para tratar la depresión), o si usted ha tomado este tipo de medicamentos en las últimas 2 semanas.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Edunix si le es aplicable cualquiera de las siguientes situaciones:
- usted o algún miembro de su familia tiene antecedentes de abuso o dependencia del alcohol, de medicamentos de venta con receta o de sustancias ilícitas (“adicción”);
- usted es fumador;
- si alguna vez ha tenido problemas con su estado de ánimo (depresión, ansiedad o un trastorno de la personalidad) o ha recibido tratamiento de un psiquiatra para otras enfermedades mentales;
- aumento de la presión cerebral o lesión en la cabeza;
- adicción al alcohol o una reacción grave a dejar de consumir alcohol (delirium tremens);
- enfermedad que causa ataques, como la epilepsia;
- un trastorno mental llamado psicosis tóxica;
- presión arterial baja (hipotensión) con bajo volumen de sangre (hipovolemia);
- disminución de la consciencia, con sensación de mareo o desmayo;
- problemas biliares, ataque biliar o de riñón (cólicos);
- inflamación del páncreas (pancreatitis);
- problemas intestinales, incluyendo inflamación, constricción u obstrucción del intestino;
- agrandamiento de la próstata, lo que provoca dificultad para orinar (hipertrofia prostática);
- función reducida de la glándula suprarrenal (por ejemplo, enfermedad de Addison). Su médico tendrá que controlar los niveles de cortisona de la sangre;
- baja actividad del tiroides (hipotiroidismo);
- problemas para respirar con normalidad (como la enfermedad pulmonar obstructiva crónica o capacidad respiratoria reducida como el asma);
- pacientes de edad avanzada o debilitados;
- problemas graves de riñón o hígado.
Si padece o ha padecido en el pasado cualquiera de las afecciones o síntomas anteriores, consulte con su médico ya que puede necesitar una dosis más baja del medicamento.
Abuso y dependencia
Hidromorfona - como otros analgésicos fuertes - tiene un perfil de abuso. El uso repetido de este medicamento puede provocar el desarrollo de dependencia psicológica o física y abuso, lo que puede provocar una sobredosis potencialmente mortal. Es importante que informe a su médico si piensa que puede haber desarrollado dependencia a este medicamento. Por lo tanto, los pacientes con antecedentes actuales o pasados de abuso de alcohol o drogas deben usar Edunix con especial precaución.
La interrupción brusca de la terapia puede causar un síndrome de abstinencia. Si ya no se necesita tratamiento con hidromorfona, puede ser aconsejable reducir gradualmente la dosis diaria para evitar los síntomas de un síndrome de abstinencia.
ToleranciaEste medicamento contiene hidromorfona, que es un opioide. El uso repetido de analgésicos opioides puede reducir la eficacia del fármaco (su organismo se acostumbra al fármaco). Esto conduce al uso de dosis más altas con el fin de lograr el alivio del dolor que se desea. Puede haber tolerancia cruzada a otros opioides; esto significa que, al tomar otro opioide, también puede habituarse a ese opioide.
Aumento de la sensibilidad al dolorCon el uso de dosis altas rara vez se puede producir un aumento de la sensibilidad al dolor (hiperalgesia), que no responderá a un aumento adicional de la dosis de hidromorfona. En este caso, su médico modificará el tratamiento individualmente.
Trastornos de la respiración relacionados con el sueño
Edunix puede causar trastornos respiratorios relacionados con el sueño como apnea del sueño (pausa de la respiración durante el sueño) e hipoxemia relacionada con el sueño (niveles bajos de oxígeno en la sangre). Los síntomas pueden incluir interrupciones de la respiración durante el sueño, despertares nocturnos debido a la dificultad para respirar, dificultad para mantener el sueño o somnolencia excesiva durante el día. Si usted u otra persona observa estos síntomas, consulte a su médico. Su médico puede considerar disminuir la dosis.
CirugíaEl uso de Edunix no se recomienda antes ni durante las primeras 24 horas después de una cirugía. Después de este tiempo Edunix debe utilizarse con precaución, sobre todo después de una cirugía abdominal.
Parálisis de la motilidad intestinal
Edunix no debe usarse en situaciones en las que pueda producirse parálisis de la motilidad intestinal (íleo paralítico). En caso que durante su uso se sospecha u ocurra íleo paralítico, el tratamiento con hidromorfona debe interrumpirse inmediatamente.
Terapia adicional para el dolorSi usted se va a someter a una terapia adicional para el dolor (por ejemplo, cirugía, bloqueo del plexo) no debe tomar hidromorfona durante las 24 horas previas a la cirugía. A partir de entonces se reajustará la dosis, de lo cual se ocupará su médico en caso necesario.
Cambiar a otro opioide
Debe tener en cuenta que, una vez que se le aplica una dosis efectiva de un opioide determinado (grupo de analgésicos potentes a los que pertenece Edunix), no se le debe cambiar a otro opioide sin una evaluación médica y una nueva evaluación cuidadosa basada en su necesidades. O bien, no se garantizará el alivio continuo del dolor.
Función insuficiente de la corteza suprarrenal
Si su corteza suprarrenal no funciona como debería (insuficiencia adrenocortical), es posible que su médico quiera controlar su concentración de cortisol en plasma y recetarle medicamentos apropiados (corticoides).
Interferencia con la producción normal de hormonas
Edunix puede interferir con la producción normal de hormonas producidas por su cuerpo (como cortisol u hormonas sexuales). Esto puede suceder especialmente después de haber tomado dosis altas durante largos períodos de tiempo.
NotaLos comprimidos de liberación prolongada nunca deben ser triturados e inyectados ya que esto podría dar lugar a efectos adversos graves, con un desenlace fatal.
Uso en deportistas
Este medicamento contiene hidromorfona que puede producir un resultado positivo en las pruebas de control de dopaje.
Niños
No se recomienda el uso de este medicamento en niños menores de 12 años. No se han realizado estudios clínicos sobre el uso de hidromorfona en niños.
Otros medicamentos y Edunix
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
El uso concomitante de Edunix y medicamentos sedantes como las benzodiacepinas (que pueden ayudar a reducir la ansiedad y las crisis, relajar los músculos e inducir el sueño) aumenta el riesgo de adormecimiento, dificultades para respirar (depresión respiratoria) y coma, y puede ser potencialmente mortal. Por lo tanto, solo se debe considerar el uso concomitante cuando no seanposibles otras opciones de tratamiento. El uso concomitante de opioides y fármacos utilizados para tratar la epilepsia, el dolor de nervio o la ansiedad (gabapentina y pregabalina) aumenta el riesgo de sobredosis por opioides y depresión respiratoria, y puede ser potencialmente mortal.
Sin embargo, si su médico le prescribe Edunix junto con medicamentos sedantes, su médico debe limitar la dosis y la duración del tratamiento concomitante.
Informe a su médico de todos los medicamentos sedantes que esté tomando y siga atentamente la dosis recomendada por su médico. Podría ser útil informar a amigos o familiares que estén al tanto de los signos y síntomas indicados anteriormente. Póngase en contacto con su médico cuando experimente dichos síntomas.
Cuando se toma junto a otros medicamentos depresores de la función cerebral o alcohol, pueden aumentar los efectos adversos de hidromorfona o del otro medicamento, por ejemplo amodorramiento o deterioro de la función respiratoria.
Tales medicamentos depresores son:
- medicamentos para tratar la ansiedad (por ejemplo tranquilizantes);
- anestésicos que relajan los músculos (como barbitúricos);
- medicamentos para tratar trastornos psiquiátricos o mentales (neurolépticos);
- medicamentos para ayudarle a dormir (como hipnóticos o sedantes);
- medicamentos para tratar la depresión (antidepresivos);
- medicamentos para tratar la alergia, el mareo o las náuseas (antihistamínicos o antieméticos);
- otros analgésicos potentes (opioides). Puede haber una tolerancia cruzada con otros opiáceos. Si además, usted toma otros analgésicos potentes (opioides) puede desarrollar una tolerancia a estos analgésicos.
No debe tomar Edunix si está tomando medicamentos para tratar la depresión llamados inhibidores de la monoamino oxidasa (IMAOs) o durante las dos semanas después de suspender estos medicamentos (ver sección 2 “No tome Edunix”).
El uso simultáneo de hidromorfona con ciertos relajantes musculares (llamados relajantes musculares, que generalmente se inyectan o toman por vía oral en forma de comprimidos) puede dar lugar a una mayor dificultad para respirar (depresión respiratoria).
Toma de Edunix con alcohol
Beber alcohol mientras esté tomando este medicamento puede hacerle sentir más somnoliento o aumentar el riesgo de reacciones adversas graves tales como respiración superficial con el riesgo de dejar de respirar, y pérdida de conocimiento. Se recomienda no beber alcohol mientras esté tomando Edunix.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No se recomienda el uso de Edunix durante el embarazo a menos que se lo haya indicado específicamente su médico. No hay o la cantidad de datos es insuficiente sobre el uso de hidromorfona en mujeres embarazadas.Si se le administra Edunix durante el embarazo, puede verse afectada la contractilidad del útero. Además, hay un riesgo de dificultad respiratoria (depresión respiratoria) en el recién nacido.
Los recién nacidos pueden sufrir síndrome de abstinencia (como llanto agudo, temblores, convulsiones, falta de apetito y diarrea) si sus madres han tomado hidromorfona de forma prolongada durante el embarazo.
Lactancia
Hidromorfona puede pasar a la leche materna. Por tanto, Edunix no debe utilizarse si está en periodo de lactancia. Si su uso es necesario, debe interrumpir la lactancia.
Conducción y uso de máquinas
Hidromorfona tiene una influencia moderada sobre la capacidad para conducir y utilizar máquinas. Estos efectos son más notables al inicio del tratamiento con hidromorfona, después de aumentar la dosis o cambiar de medicamento. Esto también es probable si combina Edunix con alcohol u otros agentes depresores del sistema nervioso central. Si usted está estabilizado con una dosis específica, puede no estar influenciado necesariamente. Por lo tanto, debe consultar a su médico por si conducir o utilizar maquinaria le estuviera permitido.
Edunix contiene sacarosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Edunix contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por comprimido de liberación prolongada; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Edunix
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis depende de la gravedad de su dolor y de las necesidades previas de analgésicos.
Adultos y adolescentes mayores de 12 años
A menos que se lo indique su médico, la dosis inicial en adultos y adolescentes mayores de 12 años de edad es de 8 mg una vez al día (cada 24 horas).
Nota importante: La dosis diaria de Edunix no debe administrarse más de una vez cada 24 horas y debe tomarse aproximadamente a la misma hora todos los días La dosis no debe incrementarse en intervalos de tiempo menores de 2 días..
Si no se logra un alivio adecuado del dolor, su médico le aumentará la dosis. En general, la dosis mínima efectiva para aliviar el dolor será elegida para cada caso individual
Edunix 32 mg comprimidos de liberación prolongada no es adecuado para un tratamiento inicial con opioides, sólo se debe utilizar en casos en los que las dosis más bajas no proporcionan suficiente alivio del dolor.
Si estima que la acción de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico.
Una vez ajustada una dosis efectiva de Edunix no se debe cambiar a otros analgésicos fuertes (preparaciones analgésicas opioides). Será necesaria la evaluación clínica y el ajuste cuidadoso de la dosis por su médico. De lo contrario, no está garantizado un alivio continuo del dolor.
Pacientes de edad avanzada
Los pacientes de edad avanzada pueden requerir una dosis menor para lograr la analgesia adecuada.
Pacientes con insuficiencia hepática o renal
Si usted padece insuficiencia renal o hepática informe de ello a su médico porque puede necesitar dosis más bajas para conseguir el control del dolor. Por tanto, su dosis de hidromorfona debe ajustarse con especial cuidado. No se recomienda la administración de este medicamento a pacientes con insuficiencia hepática grave.
Uso en niños
No se recomienda el uso de este medicamento en niños menores de 12 años. No se han realizado estudios clínicos sobre el uso de Edunix en los niños. Por lo tanto, no se puede dar una dosis recomendada para esta población de pacientes. La dosis dependerá de la intensidad del dolor y de su historial anterior en cuanto a necesidades analgésicas.
Forma de administraciónVía oral.
Trague los comprimidos de liberación prolongada con una cantidad suficiente de líquido (medio vaso de agua). Los comprimidos de liberación prolongada pueden dividirse en dosis iguales utilizando las ranuras. Los comprimidos de liberación prolongada no se deben masticar ni triturar ya que esto puede conducir a una rápida liberación de hidromorfona, y a los síntomas de una sobredosis (ver abajo "Si toma más Edunix del que debe").
Cómo abrir el blíster a prueba de niños1. Corte una sola dosis a lo largo de la línea de perforación del blíster.
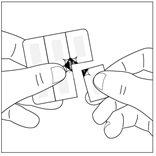
- De este modo el área no sellada es más accesible, donde están marcadas las líneas de perforación.
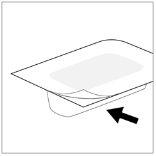
- Tire de la parte no sellada de la etiqueta para que se despegue el aluminio de cobertura.
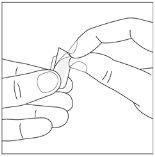
Duración del tratamiento
Usted no debe tomar este medicamento más tiempo del necesario.
Su tratamiento debe ser reevaluado regularmente con respecto al alivio del dolor y otros efectos, con el fin de lograr un tratamiento óptimo del dolor y que los efectos secundarios vayan apareciendo, así como considerar la continuación del tratamiento.
Si toma más Edunix del que debe
Si usted ha tomado más comprimidos de liberación prolongada que los recetados, debe informar a su médico de inmediato. En caso de sobredosis o ingestión accidental, consulte con su médico o llame al Servicio de Información Toxicológica; Teléfono 91 562 04 20, indicando el medicamento y la cantidad tomada.
Se pueden presentar los siguientes síntomas: pupilas contraídas(miosis), latido cardíaco lento (bradicardia), respiración deprimida (depresión respiratoria), disminución de la presión arterial (hipotensión) y aumento de adormecimiento (somnolencia) que progresa a rigidez (estupor) o pérdida de la conciencia (coma). Los pacientes pueden desarrollar neumonía (posibles síntomas: dificultad para respirar, tos y fiebre) causados por la inhalación de vómitos o sólidos.
En casos graves de colapso circulatorio o inconsciencia profunda (coma) puede ocasionar la muerte. En ningún caso debe exponerse a situaciones que requieren una concentración elevada, por ejemplo conducir un coche.
En caso de sobredosis, la siguiente medida puede ser aconsejable hasta que llegue el médico: mantenerse despierto, dar instrucciones de respiración, ayuda respiratoria.
Si olvidó tomar Edunix
No tome una dosis doble para compensar las dosis olvidadas.
Si toma una dosis menor de la indicada o si se le olvida tomar el comprimido de liberación prolongada, consecuentemente, el alivio del dolor será insuficiente o cesará por completo.Si olvidó tomar un comprimido, puede compensar la dosis de inmediato e iniciar un nuevo horario de 24 horas. En principio, no debe tomar más de 1 comprimido de Edunix comprimidos de liberación prolongada una vez cada 24 horas.
Si interrumpe el tratamiento con Edunix
No deje de tomar este medicamento sin consultar con su médico. Si usted deja de tomar Edunix después de un uso prolongado de tiempo, puede tener síntomas de abstinencia ( por ejemplo agitación, ansiedad, nerviosismo, dificultad para dormir, movimientos involuntarios, temblor y malestar estomacal/intestinal) Si la terapia ya no es necesaria, el tratamiento debe ser interrumpido gradualmente reduciendo la dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos o signos a tener en cuenta y medidas a tomar cuando se presentan estos efectos adversos o signos:
- repentinas reacciones alérgicas graves potencialmente mortales:
- reacciones de hipersensibilidad (la frecuencia no puede estimarse a partir de los datos disponibles);
- reacciones anafilácticas (la frecuencia no puede estimarse a partir de los datos disponibles).
Contacte con su médico de inmediato si nota alguno de los siguientes síntomas: dificultad para respirar, hinchazón de párpados, cara, labios, boca o garganta, erupción cutánea o picazón, especialmente aquellos que cubren todo su cuerpo.
Si usted tiene una reacción alérgica grave, deje de usar Edunix comprimidos de liberación prolongada y acuda a un médico de inmediato. Es posible que necesite tratamiento médico urgente.
La respiración lenta y débil (depresión respiratoria) es el efecto adverso más grave de la sobredosis por opioides.
La mayoría de los pacientes se estriñen si toman hidromorfona. Si tiene estreñimiento o náuseas (sensación de malestar), su médico tomará las medidas apropiadas. Usted puede contrarrestar el efecto secundario el estreñimiento mediante el uso de medidas de prevención (por ejemplo, incremento de bebida, alimentos enriquecidos en fibra como fruta, verduras y productos integrales). Si usted ya tenía problemas de estreñimiento antes de comenzar a tomar sus comprimidos de liberación prolongada, debe tomar laxantes desde el principio. Por favor, consulte a su médico.
Si usted sufre mareos o vómitos (ocurre con frecuencia sobre todo al inicio del tratamiento), su médico le puede prescribir el medicamento adecuado.
Otros posibles efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Estreñimiento, náuseas.
- Mareos, somnolencia.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Malestar.
- Reducción del apetito, pérdida de apetito.
- Ansiedad, confusión, insomnio.
- Dolor de cabeza.
- Sequedad en la boca, dolor abdominal o molestias abdominales.
- Picazón, sudoración.
- Necesidad repentina de orinar.
- Sensación de debilidad.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Síntomas de abstinencia como agitación, ansiedad, nerviosismo, dificultad para dormir, movimientos involuntarios (por ejemplo temblor) o malestar estomacal/intestinal (al parar de tomar Edunix).
- Indigestión, diarrea, trastornos del gusto .
- Hiperexcitabilidad, depresión, cambios del estado de ánimo (euforia), alucinaciones, pesadillas.
- Temblores, espasmos musculares, sensaciones anormales en la piel (pinchazo y agujas).
- Alteraciones visuales.
- Presión arterial baja.
- Dificultad para respirar.
- Cambios en los análisis de sangre que muestran cómo funciona su hígado (aumento de las enzimas hepáticas).
- Erupción cutánea, urticaria.
- Dificultad para orinar.
- Disminución del deseo sexual, impotencia.
- Cansancio, generalmente malestar, hinchazón de manos, tobillos o pies (acumulación de líquido en el tejido).
Raros (pueden afectar hasta 1 de cada 1.000 personas):
- Somnolencia hasta adormecimiento (sedación), falta de energía.
- Aumento de la frecuencia cardiaca.
- Ritmo cardíaco lento, ritmo cardíaco anormal.
- Convulsiones de la musculatura respiratoria, debilitamiento y ralentización de la respiración (depresión respiratoria), dificultad para respirar y sibilancias (broncoespasmo).
- Cambios en los análisis de sangre que muestran cómo funciona su páncreas.
- Enrojecimiento de la cara.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- Agotamiento, movimientos musculares incontrolados, aumento de la sensibilidad al dolor (hiperalgesia, ver sección 2 "Advertencias y precauciones").
- Pérdida de movimiento intestinal (íleo paralítico).
- Cólico biliar.
- Adicción al medicamento, cambios de humor (disforia), inquietud.
- Constricción de las pupilas (miosis).
- Sensación de calor.
- Erupción cutánea con picazón (urticaria).
- Necesidad de tomar dosis más altas (llamado habituación o tolerancia a las drogas).
- Apnea del sueño (interrupciones de la respiración durante el sueño).
Síndrome de abstinencia de drogas en recién nacidos cuyas madres han tomado Edunix durante el embarazo (ver sección "Embarazo y lactancia")
Si tiene algún efecto adverso, informe a su médico o farmacéutico. Esto incluye los efectos adversos no mencionados en este prospecto.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema de notificación incluido en el Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano. Website: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de ese medicamento.
5. Conservación de Edunix
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y blíster, después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. P a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deEdunix
- El principio activo es hidrocloruro de hidromorfona.
Cada comprimido de liberación prolongada contiene 32 mg de hidrocloruro de hidromorfona (equivalente a 28,38 mg de hidromorfona).
- Los demás componentes son:
Núcleo del comprimido: Esferas de azúcar, hipromelosa, etilcelulosa, hiprolosa, trietilcitrato, talco, carmelosa sódica, celulosa microcristalina, estearato de magnesio, sílice coloidal anhidra.
Recubrimiento del comprimido: Alcohol polivinílico, macrogol 4000, talco, óxido de hierro (III) (E172)
Aspecto del producto y contenido del envase
Los comprimidos de Edunix 32 mg comprimidos de liberación prolongada son rojos oscuros, oblongos, biconvexos de 18 x 8,5 mm con ranura en ambas caras.
Los comprimidos pueden dividirse en dosis iguales.
Se presentan en blísters de aluminio/PVC-PE-PVDC a prueba de niños.
Tamaños de envase: 10, 14, 20, 28, 30, 50, 56, 60, 98, 100 comprimidos de liberación prolongada.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Aristo Pharma GmbH
Wallenroder Str. 8-10
13435 Berlín
Alemania
Responsable de la fabricación
Aristo Pharma GmbH
Wallenroder Str. 8-10
13435 Berlín
Alemania
O
Laboratorios Medicamentos Internationales, S.A.
C/Solana, 26
Torrejón de Ardoz
28850 Madrid
España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid- España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania: Hydromorphon Aristo long 32 mg Retardtabletten
España: Edunix 32 mg comprimidos de liberación prolongada
Fecha de la última revisión de este prospecto: Diciembre 2022
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia151.32 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EDUNIX 32 MG COMPRIMIDOS DE LIBERACION PROLONGADAForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 16 mgPrincipio activo: hydromorphoneFabricante: Aristo Pharma GmbhRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 2,6 mg/mlPrincipio activo: hydromorphoneFabricante: Aristo Pharma GmbhRequiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 4 mgPrincipio activo: hydromorphoneFabricante: Aristo Pharma GmbhRequiere receta
Médicos online para EDUNIX 32 MG COMPRIMIDOS DE LIBERACION PROLONGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EDUNIX 32 MG COMPRIMIDOS DE LIBERACION PROLONGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















