EBGLYSS 250 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar EBGLYSS 250 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ebglyss 250mg solución inyectable en pluma precargada
lebrikizumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas. aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ebglyss y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ebglyss
- Cómo usar Ebglyss
- Posibles efectos adversos
- Conservación de Ebglyss
- Contenido del envase e información adicional
Instrucciones de uso
1. Qué es Ebglyss y para qué se utiliza
Ebglyss contiene el principio activo lebrikizumab.
Ebglyss se utiliza para tratar a adultos y adolescentes a partir de 12 años con un peso corporal no inferior a 40 kg con dermatitis atópica de moderada a grave, también conocida como eccema atópico, que pueden tratarse con tratamientos sistémicos (un medicamento administrado por boca o mediante inyección).
Ebglyss se puede administrar solo o en combinación con medicamentos para el eccema que se aplican en la piel.
Lebrikizumab es un anticuerpo monoclonal (un tipo de proteína) que bloquea la acción de otra proteína llamada interleuquina 13. La interleuquina 13 desempeña un papel importante en los síntomas de la dermatitis atópica. Al bloquear la interleuquina 13, Ebglyss puede mejorar su dermatitis atópica y reducir el picor y el dolor de la piel asociados.
2. Qué necesita saber antes de empezar a usar Ebglyss
No use Ebglyss
- si es alérgico a lebrikizumab o alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si cree que podría ser alérgico o no está seguro, consulte a su médico, farmacéutico o enfermero antes de usar Ebglyss.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Ebglyss.
Cada vez que obtenga un nuevo envase de Ebglyss, es importante que anote la fecha y el número de lote (lo encontrará en el envase después de “Lote”) y que guarde esta información en un lugar seguro.
Reacciones alérgicas
En muy raras ocasiones, este medicamento puede provocar reacciones alérgicas (hipersensibilidad). Estas reacciones pueden ocurrir poco después de empezar a tomar Ebglyss, pero también pueden darse después. Si nota que tiene síntomas de una reacción alérgica, debe dejar de usar el medicamento y ponerse en contacto con su médico o conseguir atención médica inmediatamente. Los signos de una reacción alérgica incluyen:
- problemas respiratorios
- hinchazón de la cara, la boca y la lengua
- desmayos
- mareos
- vahídos (debidos a una bajada de la tensión arterial)
- habones, picor y erupción cutánea.
Problemas oculares
Consulte a su médico en caso de aparición o empeoramiento de algún problema ocular, como enrojecimiento y malestar en el ojo, dolor en los ojos o cambios en la vista.
Vacunas
Hable con su médico respecto a su programa actual de vacunas. Véase la sección “Otros medicamentos y Ebglyss”.
Niños y adolescentes
Este medicamento no debe utilizarse en niños con dermatitis atópica menores de 12 años de edad ni en adolescentes de 12 a 17 años de edad y con un peso inferior a 40 kg, ya que no se ha evaluado en este grupo de edad.
Otros medicamentos y Ebglyss
Informe a su médico o farmacéutico si:
- está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento;
- se ha vacunado recientemente o tiene previsto hacerlo. No debe recibir determinados tipos de vacunas (vacunas vivas) durante el tratamiento con Ebglyss.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Se desconocen los efectos de este medicamento en mujeres embarazadas; es mejor evitar el uso de Ebglyss durante el embarazo a menos que el médico le aconseje utilizarlo.
Se desconoce si lebrikizumab pasa a la leche materna. Si está en periodo de lactancia o tiene intención de amamantar a un bebé, consulte a su médico antes de utilizar este medicamento. Usted y su médico deben decidir si amamantará al bebé o utilizará Ebglyss. No debería hacer ambas cosas.
Conducción y uso de máquinas
Es poco probable que Ebglyss influya en su capacidad para conducir y utilizar máquinas.
3. Cómo usar Ebglyss
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto Ebglyss se debe administrar y durante cuánto tiempo
Su médico decidirá cuánto Ebglyss necesita y durante cuánto tiempo lo va a utilizar.
La dosis recomendada es:
- Dos inyecciones iniciales de 250 mg de lebrikizumab cada una (500 mg en total) en la semana 0 y la semana 2.
- Una inyección con 250 mg una vez cada dos semanas desde la semana 4 hasta la semana 16.
En función de cómo responda al medicamento, su médico podrá decidir dejar de darle el medicamento o seguir dándole una inyección de 250 mg cada dos semanas hasta la semana 24.
- Una inyección con 250 mg cada cuatro semanas a partir de la semana 16 en adelante (posología de mantenimiento).
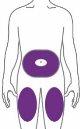
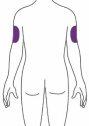
Ebglyss se administra mediante una inyección subcutánea (bajo la piel) en el muslo o el abdomen, excepto en los 5 cm alrededor del ombligo. Si otra persona administra la inyección, también se puede administrar en la parte superior del brazo. Usted y su médico o enfermero decidirán si puede inyectarse Ebglyss usted mismo.
Se recomienda que alterne el lugar de la inyección con cada inyección. Ebglyss no se debe inyectar en zonas de piel sensible, dañada o con hematomas o cicatrices, ni en zonas de piel afectada por la dermatitis atópica u otras lesiones cutáneas. La dosis inicial de 500 mg se debe administrar en dos inyecciones consecutivas de 250 mg en diferentes lugares de inyección.
Es importante que no intente ponerse la inyección usted mismo hasta que su médico o enfermero le hayan enseñado cómo hacerlo. También es posible que le administre las inyecciones de Ebglyss un cuidador que haya aprendido a hacerlo bien. En el caso de los adolescentes, de 12 años de edad o más, se recomienda que Ebglyss lo administre un adulto o este se administre bajo la supervisión de un adulto.
La pluma precargada no se debe agitar.
Lea detenidamente las “Instrucciones de uso” de la pluma precargada antes de administrar Ebglyss.
Si usa más Ebglyss del que debe
Si utiliza más Ebglyss del que le recetó su médico o se administra la dosis antes de lo previsto, hable con su médico, farmacéutico o enfermero.
Si olvidó usar Ebglyss
Si ha olvidado inyectarse una dosis de Ebglyss, consulte a su médico, farmacéutico o enfermero.
Si olvidó inyectarse Ebglyss cuando tiene previsto hacerlo usualmente, adminístrese la inyección cuando se acuerde de ello. La siguiente dosis se debe inyectar con normalidad el día que corresponda.
Si interrumpe el tratamiento con Ebglyss
No deje de usar Ebglyss sin hablar primero con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Enrojecimiento y molestias en el ojo (conjuntivitis)
- Inflamación del ojo debido a una reacción alérgica (conjuntivitis alérgica)
- Ojo seco
- Reacciones en el lugar de la inyección
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Herpes zóster (culebrilla), erupción con ampollas muy dolorosa en una parte del cuerpo
- Aumento de eosinófilos (un tipo de glóbulos blancos, eosinofilia)
- Inflamación de la córnea (la capa transparente que cubre la parte delantera del ojo, queratitis)
- Picor, enrojecimiento e hinchazón de párpados (blefaritis)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ebglyss
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento si observa que la solución está turbia o ha cambiado de color o si contiene partículas visibles. Antes del uso, saque la caja de la nevera, saque la pluma precargada de la caja y espere 45 minutos a que alcance la temperatura ambiente. Después de sacarlo de la nevera, Ebglyss se debe conservar por debajo de 30 °C y utilizar en un plazo de 7 días o desechar. Una vez conservado fuera de la nevera, no se puede volver a refrigerar. Puede anotar la fecha en que lo sacó de la nevera en la caja del medicamento.
Este medicamento es de un solo uso.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ebglyss
- El principio activo es lebrikizumab. Cada pluma precargada contiene 250 mg de lebrikizumab en 2 ml de solución (125 mg/ml).
- Los demás componentes son histidina, ácido acético glacial (E260), sacarosa, polisorbato 20 (E432) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ebglyss es una solución inyectable estéril de transparente a opalescente, entre incolora a ligeramente amarilla o/a ligeramente marrón y sin partículas visibles. Se suministra en envases de cartón que contienen una pluma precargada unidosis o 2 plumas precargadas unidosis, y en envases múltiples que contienen 3 plumas precargadas unidosis (3 envases de 1), 4 plumas precargadas unidosis (2 envases de 2), 5 plumas precargadas unidosis (5 envases de 1) o 6 plumas precargadas unidosis (3 envases de 2). Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Almirall, S.A.
Ronda General Mitre, 151
08022 Barcelona
España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
España
Almirall, S.A.
Tel: +34 93 291 30 00
Fecha de la última revisión de este prospecto: 11/2023.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
Instrucciones de uso
Estas Instrucciones de uso contienen información sobre cómo administrar las inyecciones de Ebglyss. |
Lea estas “Instrucciones de uso” antes de la utilización de este medicamento y siga atentamente todas las instrucciones paso a paso. |
|
Información importante que necesita conocer antes de inyectar Ebglyss |
|
|
|
|
|
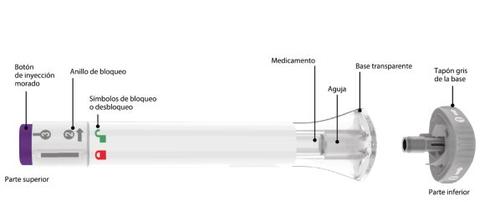
Partes de la pluma precargada de Ebglyss |
|
Preparación para la inyección de Ebglyss | ||
Prepare los suministros: | ||
|
| |
Espere 45 minutos | ||
Saque la pluma precargada de Ebglyss de la caja con el tapón gris de la base puesto y deje que la pluma precargada se atempere durante 45 minutos a temperatura ambiente antes de la inyección.
| ||
Inspeccione la pluma precargada y el medicamento | ||
Asegúrese de que tenga el medicamento correcto. El medicamento contenido en el interior de la pluma debe ser transparente. Puede ser entre incoloro a ligeramente amarillo o/a ligeramente marrón. | ||
| Noutilice la pluma precargada (consulte la sección Eliminación de la pluma precargada de Ebglyss) si:
| |
Lávese las manos con agua y jabón | ||
Seleccione y limpie el lugar de la inyección | ||
Su médico puede ayudarle a elegir el lugar de la inyección que más le convenga. Limpie el lugar de la inyección con una toallita impregnada en alcohol y déjelo secar. | ||
| Usted u otra persona, puede inyectar, o le pueden inyectar el medicamento, en estas zonas. |
Al menos a 5 cm del ombligo.
Al menos 5 cm por encima de la rodilla y 5 cm por debajo de la ingle. |
| Otra persona debe inyectar el medicamento en esta zona. |
Otra persona debe inyectar en la cara externa del brazo. Nose debe inyectar cada vez en el mismo punto exactamente. No se debe inyectaren zonas de piel sensible, con hematomas, enrojecida, dura o con cicatrices, ni en zonas de piel afectadas por la dermatitis atópica u otras lesiones cutáneas. |
Inyección de Ebglyss | ||||
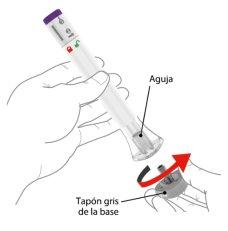
1 | Destape la pluma precargada |
| ||
| Asegúrese de que la pluma precargada esté bloqueada. | |||
Cuando esté listo para administrarse la inyección, gire el tapón gris de la base y tírelo a la basura doméstica. Novuelva a colocar el tapón gris de la base, ya que podría dañar la aguja. Notoque la aguja dentro de la base transparente. | ||||
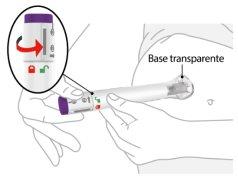
2 | Colóquela y desbloquee | |||
| Coloquela base transparente contra la piel y sujétela firmemente. | |||
Mantenga la base transparente sobre la piel y, a continuación, gire el anillo de bloqueo hasta la posición de desbloqueo. | ||||
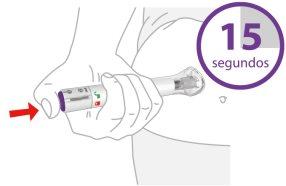
3 | Pulse y mantenga pulsado durante 15 segundos | |||
| Pulseel botón de inyección morado, manténgalo pulsadoy espere a escuchardos clics fuertes:
La inyección puede durar hasta 15 segundos. | |||
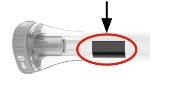
Sabrá que la inyección ha finalizado cuando el émbolo gris sea visible. A continuación, retire la pluma precargada del lugar de la inyección. |
| Émbolo gris |
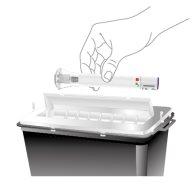
Eliminación de la pluma precargada de Ebglyss | |
Deseche la pluma precargada usada | |
| Deseche la pluma precargada de Ebglyss usada en un recipiente para objetos punzantes inmediatamente después del uso. |
No tire (deseche) la pluma precargada de Ebglyss a la basura doméstica. | |
Si no tiene un recipiente para objetos punzantes, puede usar un recipiente doméstico que:
| |
Cuando el recipiente para objetos punzantes esté casi lleno, tendrá que seguir las directrices de su localidad para su correcta eliminación. Es posible que existan leyes nacionales sobre la eliminación de agujas y jeringas. Para obtener más información sobre la eliminación segura de objetos punzantes, pregunte a su médico sobre las opciones disponibles en su localidad. Norecicle el recipiente usado para objetos punzantes. | |
Preguntas frecuentes | |
P. | ¿Qué sucede si hay burbujas en la pluma precargada? |
R. | Es normal que haya burbujas de aire. No le dañarán ni afectarán a su dosis. |
P. | ¿Qué ocurre si hay una gota de líquido en la punta de la aguja al quitar el tapón gris de la base? |
R. | Es normal que haya una gota de líquido en la punta de la aguja. No le dañará ni afectará a su dosis. |
P. | ¿Qué ocurre si desbloqueo la pluma y pulso el botón de inyección morado antes de haber girado el tapón gris de la base? |
R. | Noquite el tapón gris de la base. Deseche (tire) la pluma precargada y utilice una nueva. |
P. | ¿Debo mantener pulsado el botón de inyección morado hasta que se haya completado la inyección? |
R. | No es necesario mantener pulsado el botón de inyección morado, pero puede ayudarle a mantener la pluma precargada estable y firme contra la piel. |
P. | ¿Qué ocurre si la aguja no se retrae después de la inyección? |
R. | Notoque la aguja ni vuelva a poner el tapón gris de la base. Guarde la pluma precargada en un lugar seguro para evitar un pinchazo accidental con la aguja. |
P. | ¿Qué sucede si tengo una gota de líquido o de sangre en la piel después de la inyección? |
R. | Eso es algo normal. Haga presión sobre el lugar de la inyección con una bola de algodón o una gasa. Nofrote el lugar de la inyección. |
P. | ¿Cómo puedo saber si mi inyección ha finalizado? |
R. | Después de pulsar el botón de inyección morado, oirá 2 clics fuertes. El segundo clic fuerte indica que la inyección ha finalizado. También verá el émbolo gris en la parte superior de la base transparente. La inyección puede durar hasta 15 segundos. |
P. | ¿Qué ocurre si retiro la pluma precargada antes del segundo clic fuerte o antes de que el émbolo gris haya dejado de moverse? |
R. | Es posible que no haya recibido la dosis completa. No se administre otra inyección. Llame a su médico para que le ayude. |
P. | ¿Qué ocurre si he oído más de 2clics durante la inyección? (2 clics fuertes y 1 clic suave). ¿He recibido la inyección completa? |
R. | Algunas personas pueden oír un clic suave justo antes del segundo clic fuerte. Este es el funcionamiento normal de la pluma precargada. Noretire la pluma precargada de la piel hasta que oiga el segundo clic fuerte. |
Lea el prospecto completo de la jeringa precargada antes de usar Ebglyss.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EBGLYSS 250 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 250 mgPrincipio activo: LebrikizumabFabricante: Almirall S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1 mlPrincipio activo: TralokinumabFabricante: Leo Pharma A/SRequiere recetaForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: TralokinumabFabricante: Leo Pharma A/SRequiere receta
Médicos online para EBGLYSS 250 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EBGLYSS 250 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes