
DORZOLAMIDA/TIMOLOL AUROVITAS 20mg/ml + 5 mg/ml COLIRIO EN SOLUCION


Cómo usar DORZOLAMIDA/TIMOLOL AUROVITAS 20mg/ml + 5 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Dorzolamida/Timolol Aurovitas y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dorzolamida/Timolol Aurovitas
- Cómo usar Dorzolamida/Timolol Aurovitas
- Posibles efectos adversos
- Conservación de Dorzolamida/Timolol Aurovitas
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Dorzolamida/Timolol Aurovitas 20mg/ml + 5mg/ml colirio en solución
Dorzolamida/Timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Dorzolamida/Timolol Aurovitas y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dorzolamida/Timolol Aurovitas
- Cómo usar Dorzolamida/Timolol Aurovitas
- Posibles efectos adversos
- Conservación de Dorzolamida/Timolol Aurovitas
- Contenido del envase e información adicional
1. Qué es Dorzolamida/Timolol Aurovitas y para qué se utiliza
Dorzolamida/Timolol Aurovitas es una combinación de dos medicamentos: dorzolamida y timolol.
Dorzolamida pertenece a un grupo de medicamentos llamados "inhibidores de la anhidrasa carbónica".
Timolol pertenece a un grupo de medicamentos llamados "betabloqueantes".
Estos medicamentos disminuyen la presión en el ojo de distintas maneras.
Dorzolamida/timolol se prescribe para reducir la presión elevada del ojo en el tratamiento del glaucoma cuando el uso de un colirio betabloqueante solo no sea adecuado.
2. Qué necesita saber antes de empezar a usar Dorzolamida/Timolol Aurovitas
No use Dorzolamida/Timolol Aurovitas:
- si es alérgico a dorzolamida hidrocloruro, timolol maleato, betabloqueantes o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene ahora o ha tenido en el pasado problemas respiratorios como asma, bronquitis obstructiva crónica grave (enfermedad pulmonar grave que puede causar pitos, dificultad para respirar y/o tos desde hace mucho tiempo),
- si sufre enfermedad o trastornos del riñón graves o tiene antecedentes de piedras en el riñón,
- si tiene exceso de acidez de la sangre causada por un acúmulo de cloruros en la sangre (acidosis hiperclorémica,
- si tiene ciertos problemas cardiacos, incluyendo ciertas alteraciones del ritmo del corazón que pueden producir un latido del corazón anormalmente lento o insuficiencia cardiaca grave.
Si no está seguro si debe usar este medicamento, consulte a su médico o farmacéutico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar dorzolamida/timolol.
Consulte a su médico antes de empezar a usar este medicamento, si padece o ha padecido en el pasado:
- enfermedad cardiaca coronaria (síntomas que pueden incluir dolor de pecho u opresión, quedarse sin respiración o asfixia), fallo cardiaco, presión arterial baja,
- alteraciones del ritmo cardiaco como latido lento del corazón,
- problemas respiratorios, asma o enfermedad pulmonar obstructiva crónica,
- enfermedad por la que tiene una pobre circulación de la sangre (como enfermedad de Raynaud o síndrome de Raynaud),
- diabetes, ya que Dorzolamida/Timolol Aurovitas puede enmascarar los signos y síntomas de una bajada de azúcar en sangre,
- hiperactividad de la glándula tiroides debido a que Dorzolamida/Timolol Aurovitas puede enmascarar sus signos y síntomas,
Informe a su médico, antes de que tenga una intervención quirúrgica, que está usando dorzolamida/timolol, ya que el timolol puede cambiar los efectos de algunos medicamentos usados durante la anestesia.
Informe también a su médico acerca de cualquier alergia o reacción alérgica, como urticaria, hinchazón de la cara, los labios, la lengua y/o la garganta, que pueden causar dificultad para respirar o tragar.
Informe a su médico si tiene debilidad muscular o si ha sido diagnosticado de miastenia gravis,
Si desarrolla cualquier irritación ocular o cualquier problema nuevo en los ojos, como enrojecimiento de los ojos o hinchazón de los párpados, informe a su médico inmediatamente.
Si sospecha que dorzolamida/timolol le está causando una reacción alérgica o hipersensibilidad (por ejemplo, erupción cutánea, reacción cutánea grave, o enrojecimiento y picor en el ojo), deje de utilizar este medicamento y consulte inmediatamente a su médico.
Informe a su médico si se produce una infección ocular, si sufre una lesión en el ojo, si se somete a una intervención quirúrgica ocular o si se desarrollan otras reacciones o un empeoramiento de los síntomas.
Cuando se instila dorzolamida/timolol en el ojo, puede afectar a todo el cuerpo.
Si usa lentes de contacto blandas, debe consultar a su médico antes de usar este medicamento.
Niños y adolescentes
Se dispone de datos clínicos limitados de la administración de Dorzolamida/Timolol Aurovitas en lactantes y en niños.
Uso en pacientes de edad avanzada
En estudios con dorzolamida/timolol colirio en solución, los efectos de dorzolamida/timolol colirio en solución fueron similares tanto en pacientes de edad avanzada como en pacientes más jóvenes.
Uso en pacientes con insuficiencia hepática
Informe a su médico si sufre o ha sufrido problemas del hígado.
Uso en deportistas
Se debe advertir a los pacientes que este medicamento contiene timolol, que puede producir un resultado positivo en las pruebas de control del dopaje.
Otros medicamentos y Dorzolamida/Timolol Aurovitas
Dorzolamida/Timolol Aurovitas puede afectar o ser afectado por otros medicamentos que esté utilizando, incluyendo otros colirios para el tratamiento del glaucoma. Informe a su médico si está usando o tiene intención de usar medicamentos para reducir la presión arterial, medicamentos para el corazón o medicamentos para tratar la diabetes. Informe a su médico o farmacéutico que está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. Esto es particularmente importante si está:
- Tomando medicamentos antihipertensivos que se utilizan para reducir la presión arterial o para tratar enfermedades del corazón tales (como bloqueantes de los canales de calcio y betabloqueantes o digoxina).
- Tomando medicamentos para tratar una alteración o irregularidad del latido del corazón tales como bloqueadores de los canales de calcio, betabloqueantes o digoxina.
- Usando otro colirio que contiene un betabloqueante.
- Tomando otro inhibidor de la anhidrasa carbónica como acetazolamida.
- Tomando inhibidores de la monoamino oxidasa (IMAOs).
- Tomando un medicamento parasimpaticomimético que pudiera haber sido prescrito para ayudarle a eliminar orina. Los parasimpaticomiméticos son también un tipo particular de medicamento que en algunas ocasiones se utiliza para ayudar a restaurar los movimientos normales a través del intestino.
- Tomando narcóticos tales como morfina, utilizada para tratar el dolor de moderado a agudo.
- Tomando medicamentos para tratar la diabetes.
- Tomando antidepresivos conocidos como fluoxetina y paroxetina.
- Tomando un medicamento a base de sulfa.
- Tomando quinidina (utilizada para tratar enfermedades del corazón y algunos tipos de malaria).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Uso en embarazo
No utilice Dorzolamida/Timolol Aurovitas si está embarazada a no ser que su médico lo considere necesario.
Uso en lactancia
No utilice Dorzolamida/Timolol Aurovitas si está en periodo de lactancia. Timolol puede pasar a su leche materna.
Conducción y uso de máquinas
No se han realizado estudios de los efectos sobre la capacidad para conducir o utilizar máquinas. Hay efectos adversos asociados con dorzolamida/timolol, tales como visión borrosa, que pueden afectar a su capacidad para conducir y/o manejar máquinas. No conducir o manejar máquinas hasta que se sienta bien su visión sea clara.
Dorzolamida/Timolol Aurovitas contiene cloruro de benzalconio
El cloruro de benzalconio se puede absorber por las lentes de contacto blandas y puede alterar el color de las lentes de contacto. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento.
Dorzolamida/Timolol Apotex contiene sodio:
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por frasco; esto es, esencialmente “exento de sodio”.
3. Cómo usar Dorzolamida/Timolol Aurovitas
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico si tiene dudas.
La dosis apropiada y la duración del tratamiento serán establecidas por su médico.
La dosis recomendada es una gota en el ojo u ojos afectados dos veces al día, por ejemplo por la mañana y por la noche.
Si utiliza Dorzolamida/Timolol Aurovitas al mismo tiempo que otro colirio, deje al menos 10 minutos entre la aplicación de Dorzolamida/Timolol Aurovitas y la del otro medicamento.
No cambie la dosis del medicamento sin consultar al médico. Si debe interrumpir el tratamiento, consulte a su médico inmediatamente.
No deje que la punta del recipiente toque su ojo o las zonas que lo rodean. Puede contaminarse con bacterias que pueden causar infecciones en los ojos que originen graves daños en los ojos, incluso la pérdida de la visión. Para evitar una posible contaminación del recipiente, evite que la punta del recipiente entre en contacto con cualquier superficie.
Con el fin de asegurar una dosis correcta – la punta del gotero no debe agrandarse.
Instrucciones de uso:
Se recomienda que se lave las manos antes de usar el colirio.
Puede ser más fácil la aplicación del colirio enfrente de un espejo.
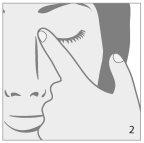
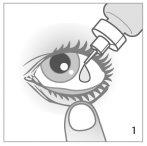
- Antes de utilizar el medicamento por primera vez, asegúrese de que la tira de seguridad en el cuello del frasco está intacta. Cuando el frasco no se ha abierto aún, es normal la existencia de un espacio entre el frasco y la tapa.
- Quite la tapa del frasco.
- Incline la cabeza hacia atrás y separe el párpado inferior ligeramente, formando una pequeña separación entre el párpado y el ojo (ver dibujo 1).
- Invierta el frasco, y presione hasta dispensar una sola gota en el ojo. NO TOQUE EL OJO NI EL PÁRPADO CON LA PUNTA DEL GOTERO (ver dibujo 1).
- Cierre el ojo y presione con el dedo la esquina interna del ojo con su dedo durante
aproximadamente dos minutos. Esto ayuda a evitar que el medicamento llegue al resto del
cuerpo. (ver dibujo 2).
- Repita los pasos 3 a 5 con el otro ojo si su médico se lo ha indicado.
- Vuelva a colocar la tapa y cierre el frasco inmediatamente después de haberlo utilizado.
Si usa másDorzolamida/Timolol Aurovitasdel que debe
Es importante que mantenga la dosis que su médico le haya indicado. Si se aplican demasiadas gotas en el ojo o traga algo del contenido del frasco, puede sentirse mal, por ejemplo puede sentir aturdimiento, tener dificultad para respirar, o notar que el corazón le late más despacio. Si siente alguno de estos efectos debe buscar atención médica inmediatamente.
En caso de sobredosis o ingestión accidental, acuda a un Centro Médico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad utilizada. Se recomienda llevar el envase y el prospecto del medicamento.
Si olvidó usarDorzolamida/Timolol Aurovitas
Es importante usar Dorzolamida/Timolol Aurovitas como le ha indicado su médico.
Si olvida aplicar una dosis, debe administrársela lo antes posible. Sin embargo, si es casi la hora de la siguiente dosis, no se administre la dosis olvidada y continúe con el programa de dosis habitual.
No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento conDorzolamida/Timolol Aurovitas
Si quiere interrumpir el uso de este medicamento consulte primero a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Generalmente puede seguir utilizando el colirio, a no ser que los efectos sean graves. Si está preocupado, hable con su médico o farmacéutico. No deje de utilizar Dorzolamida/Timolol Aurovitas sin hablar con su médico.
Si experimenta cualquiera de los siguientes efectos adversos graves,deje de usar este medicamento e informe a su médico inmediatamente o acuda a urgencias del hospital más cercano.
- Reacciones alérgicas generalizadas incluyendo hinchazón debajo de la piel que puede producirse en zonas como la cara y las extremidades, y que puede obstruir las vías respiratorias, lo que puede causar dificultad en la respiración o al tragar, urticaria o erupción con picor, erupción localizada y generalizada, picazón, reacción alérgica repentina grave que puede poner en peligro la vida.
- Enfermedad grave con descamación intensa e hinchazón de la piel, formación de ampollas en la piel, boca, ojos, genitales y fiebre. Erupción cutánea con manchas rosas-rojas especialmente en las palmas de las manos o plantas de los pies que pueden ampollarse.
Al igual que otros medicamentos que se aplican en los ojos, Dorzolamida/Timolol Aurovitas se absorbe por la sangre. Esto puede causar efectos adversos similares a los observados con agentes betabloqueantes administrados por vía “oral” y/o “intravenosa”. La incidencia de efectos adversos después de la administración tópica oftálmica es más baja que cuando los medicamentos se toman, por ejemplo, por vía oral o inyectados.
La frecuencia de los posibles efectos secundarios enumerados a continuación se define utilizando la siguiente convención:
Muy frecuentes (afecta a más de 1 de cada 10 personas)
Frecuentes (afecta de 1 a 10 personas de cada 100)
Poco frecuentes (afecta de 1 a 10 personas de cada 1000)
Raro (afecta de 1 a 10 personas en 10,000)
Frecuencia no conocida (no se puede estimar la frecuencia a partir de los datos disponibles)
Se han notificado las siguientes reacciones adversas con dorzolamida/timolol o uno de sus omponentes durante los ensayos clínicos o durante la experiencia posterior a la comercialización:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
Quemazón y escozor de los ojos, alteración del sabor
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 personas):
Enrojecimiento del ojo u ojos y alrededor, lagrimeo o picor del ojo u ojos, erosión de la córnea (daño en la capa delantera del globo ocular), inflamación y/o irritación del ojo u ojos y alrededor, sensación de tener algo en el ojo, disminución de la sensibilidad de la córnea (no aprecia que tiene algo en el ojo y no siente dolor), dolor ocular, ojos secos, visión borrosa, dolor de cabeza, sinusitis (sensación de tensión o congestión en la nariz), debilidad/cansancio y fatiga.
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
Mareos, depresión, inflamación del iris, alteraciones de la visión incluidas las modificaciones de la refracción (en algunos casos debido a la suspensión de la terapia miótica), disminución de los latidos del corazón, desvanecimiento, falta de aliento, indigestión y piedras en el riñón.
Efectos adversos raros(pueden afectar hasta a 1 de cada 1.000 personas):
Lupus eritematoso sistémico (una enfermedad inmune que puede causar una inflamación de los órganos internos), hormigueo o entumecimiento de las manos o los pies, insomnio, pesadillas, pérdida de memoria, incremento en los signos y síntomas de mistenia gravis (trastorno muscular), disminución del deseo sexual, accidente cerebro vascular, miopía transitoria que puede resolverse al cesar el tratamiento, desprendimiento de la capa inferior de la retina que contiene vasos sanguíneos después de la cirugía de filtración que puede causar alteraciones visuales, párpados caídos (haciendo que el ojo permanezca medio cerrado), visión doble, costras en el párpado, hinchazón de la córnea (con síntomas de alteraciones visuales), presión baja en el ojo, sonidos de campanilleo en el oído, presión arterial baja, cambios en el ritmo o la velocidad de los latidos del corazón, dolor del pecho, palpitaciones (latidos del corazón más rápidos y/o irregulares), infarto de miocardio, fenómeno de Raynaud, insuficiencia cardiaca congestiva (enfermedad del corazón con falta de aliento e hinchazón de los pies y las piernas debido a acumulación de líquido), edema (acumulación de líquido), isquemia cerebral (reducción del flujo sanguíneo al cerebro), manos y pies hinchados o fríos y disminución de la circulación en sus brazos o piernas, calambres en las piernas y/o dolor en las piernas cuando se camina (claudicación), falta de respiración, deterioro de la función pulmonar, goteo o congestión de nariz, hemorragia nasal, constricción de las vías respiratorias en los pulmones, tos, irritación de la garganta, sequedad de boca, diarrea, dermatitis de contacto, pérdida de pelo, erupción cutánea con apariencia de color blanco plateado (erupción psoriasiforme), enfermedad de Peyronie (que puede causar una curvatura del pene), reacciones de tipo alérgico tales como erupción cutánea, urticaria, picor, en raros casos posible hinchazón de los labios, ojos y boca, jadeo, o reacciones cutáneas graves (síndrome de Stevens Johnson, necrólisis epidérmica tóxica).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
Niveles bajos de glucosa en sangre, fallo cardiaco, trastorno del ritmo cardiaco, dolor abdominal, vómitos, dolor muscular no producido por ejercicio, disfunción sexual.
Dificultad respiratoria, sensación de cuerpo extraño en el ojo (sensación de tener algo en el ojo).
Alucinaciones, aumento de la frecuencia cardíaca, aumento de la tensión arterial.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico, particularmente si experimenta cualquier cambio o trastorno visual cuando utilice Dorzolamida/Timolol Aurovitas tras cirugía ocular.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Dorzolamida/Timolol Aurovitas
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación.
Dorzolamida/Timolol Aurovitas debe utilizarse en los 28 días despues de la primera apertura del frasco. Por lo tanto, debe desechar el frasco 4 semanas después de la primera apertura, incluso si queda colirio. Para ayudarle a recordarlo, escriba la fecha en la que abrió el frasco en el espacio del cartonaje preparado para ello.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Dorzolamida/Timolol Aurovitas
- Los principios activos son dorzolamida y timolol. Cada ml contiene 20 mg de dorzolamida (como 22,26 mg de dorzolamida hidrocloruro) y 5 mg de timolol (como 6,83 mg de timolol maleato).
- Los demás componentes son manitol (E421), hidroxietil celulosa, cloruro de benzalconio (como conservante), citrato de sodio (E331), hidróxido de sodio (E524) para ajuste del pH y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Este medicamento es un colirio en solución acuoso, estéril, incoloro, transparente, ligeramente viscoso.
Dorzolamida/Timolol Aurovitas se presenta en un frasco opaco de color blanco de polietileno de densidad media con un gotero de punta sellada de polietileno de baja densidad y una tapa de rosca de polietileno de alta densidad con una tira de seguridad. Cada frasco contiene 5 ml de solución oftálmica.
Dorzolamida/Timolol Aurovitas está disponible en envases con 1, 3 ó 6 frascos de 5 ml cada uno.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Aurovitas Spain, S.A.U.
Avda. de Burgos, 16-D
28036 Madrid
España
Responsable de la fabricación
Pharmathen S.A.
6 Dervenakion str.
15351 Pallini, Attiki
Grecia
O
Famar S.A., Plant A
63 Agiou Dimitriou Street
174 56 Alimos
Grecia
Fecha de la última revisión de este prospecto:enero 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Precio medio en farmacia12.11 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DORZOLAMIDA/TIMOLOL AUROVITAS 20mg/ml + 5 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 10 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml+5mg/mlPrincipio activo: timolol, combinationsFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para DORZOLAMIDA/TIMOLOL AUROVITAS 20mg/ml + 5 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DORZOLAMIDA/TIMOLOL AUROVITAS 20mg/ml + 5 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















