
ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ

Спросите врача о рецепте на ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ

Инструкция по применению ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ
Введение
Прошпект: информация для пациента
Дабигатран этексилатСАГ 150 мг твердые желатиновые капсулы ЕФГ
Прочитайте внимательно весь прошпект перед началом приема этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если они имеют те же симптомы, что и вы, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Содержание прошпекта
- Что такое Дабигатран этексилат САГ и для чего он используется
- Что нужно знать перед началом приема Дабигатрана этексилата САГ
- Как принимать Дабигатран этексилат САГ
- Возможные побочные эффекты
- Хранение Дабигатрана этексилата САГ
- Содержание упаковки и дополнительная информация
1. Что такое Дабигатран этексилат САГ и для чего он используется
Дабигатран этексилат САГ содержит активное вещество дабигатран этексилат и относится к группе лекарств, называемых антикоагулянтами. Он работает, блокируя вещество в организме, участвующее в образовании тромбов.
Дабигатран этексилат используется у взрослых для:
- предотвращения образования тромбов в мозге (инсульта) и в других кровеносных сосудах организма, если у вас есть форма нерегулярного сердечного ритма, называемая неклапанной фибрилляцией предсердий, и у вас есть хотя бы один дополнительный фактор риска.
- лечения тромбов в венах ног и легких и для предотвращения повторного образования тромбов в венах ног и легких.
Дабигатран этексилат используется у детей для:
- лечения тромбов и предотвращения повторного образования тромбов.
2. Что нужно знать перед началом приема Дабигатрана этексилата САГ
Не принимайте Дабигатран этексилат САГ
- если вы аллергичны к дабигатрану этексилату или к любому другому компоненту этого лекарства (перечисленному в разделе 6).
- если ваша функция почек сильно снижена.
- если у вас сейчас есть кровотечение.
- если у вас есть заболевание органа, которое увеличивает риск тяжелого кровотечения (например, язва желудка, повреждение или кровотечение мозга, недавняя операция на мозге или глазах).
- если вы склонны к кровотечению. Эта склонность может быть врожденной, неизвестной или вызванной другими лекарствами.
- если вы принимаете лекарства для предотвращения образования тромбов в крови (например, варфарин, ривароксабан, апиксабан или гепарин), за исключением случаев, когда вы меняете антикоагулянтную терапию, когда у вас есть венозный или артериальный катетер и вам вводится гепарин через этот катетер для поддержания его открытия, или когда ваш нормальный сердечный ритм восстанавливается с помощью процедуры, называемой катетерной абляцией для фибрилляции предсердий.
- если функция вашей печени сильно снижена или у вас есть заболевание печени, которое может быть смертельным.
- если вы принимаете пероральный кетоконазол или итраконазол, лекарства, используемые для лечения грибковых инфекций.
- если вы принимаете пероральный циклоспорин, лекарство, используемое для предотвращения отторжения трансплантированных органов.
- если вы принимаете дронедарон, лекарство, используемое для лечения нерегулярного сердечного ритма.
- если вы принимаете комбинированный препарат глекапревира и пибрентасвира, антивирусное лекарство, используемое для лечения гепатита С.
- если у вас имплантирована искусственная сердечная клапан, требующая постоянной антикоагулянтной терапии.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом перед началом приема дабигатрана этексилата. Во время лечения этим лекарством вам также может потребоваться проконсультироваться с вашим врачом, если вы испытываете какие-либо симптомы или если вам необходимо пройти операцию.
Сообщите вашему врачу, если у вас есть или были какие-либо расстройства или заболевания, особенно любые из следующих:
- Если у вас повышен риск кровотечения, например:
- если вы недавно имели кровотечение.
- если вы прошли хирургическую биопсию в течение последнего месяца.
- если вы имели тяжелую травму (например, перелом кости, травма головы или любая травма, требующая хирургического лечения).
- если у вас есть воспаление пищевода или желудка.
- если у вас есть проблемы с рефлюксом желудочного сока в пищевод.
- если вы принимаете лекарства, которые могут увеличить риск кровотечения. См. "Другие лекарства и Дабигатран этексилат САГ" ниже.
- если вы используете противовоспалительные лекарства, такие как диклофенак, ибупрофен или пиросикам.
- если у вас есть бактериальный эндокардит (инфекция сердца).
- если вы знаете, что у вас снижена функция почек, или если вы страдаете обезвоживанием (симптомы включают чувство жажды и выделение небольших количеств темной мочи).
- если вам больше 75 лет.
- если вы взрослый пациент и весите 50 кг или меньше.
- только если используется у детей: если у ребенка есть инфекция мозга или вокруг него.
- Если вы имели сердечный приступ или если у вас диагностировано заболевание, которое увеличивает риск сердечного приступа.
- Если у вас есть заболевание печени, связанное с изменениями в анализах крови. Применение этого лекарства не рекомендуется в этом случае.
Будьте особенно осторожны с Дабигатраном этексилатом САГ
- Если вам необходимо пройти операцию:
В этом случае дабигатран этексилат должен быть временно прекращен из-за повышенного риска кровотечения во время и после операции. Очень важно принимать дабигатран этексилат до и после операции именно в те моменты, которые указал ваш врач.
- Если операция требует установки катетера или инъекции в позвоночник (например, для эпидуральной или спинальной анестезии или для обезболивания):
- Очень важно принимать дабигатран этексилат до и после операции именно в те моменты, которые указал ваш врач.
- Сообщите вашему врачу немедленно, если у вас出现ят онемение или слабость в ногах или проблемы с кишечником или мочевым пузырем после окончания анестезии, поскольку это требует срочного внимания.
- Если вы падаете или травмируетесь во время лечения, особенно если вы ударили голову. Проконсультируйтесь с врачом немедленно. Вам может потребоваться осмотр врача, поскольку у вас может быть повышен риск кровотечения.
- Если вы знаете, что у вас есть заболевание, называемое антифосфолипидным синдромом (расстройство иммунной системы, которое увеличивает риск образования тромбов), сообщите вашему врачу, чтобы он решил, нужно ли изменить лечение.
Другие лекарства и Дабигатран этексилат САГ
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство. В частности, вы должны сообщить вашему врачу перед началом приема дабигатрана этексилата, если вы принимаете любое из следующих лекарств:
- Лекарства для снижения свертываемости крови (например, варфарин, фенпрокумон, аценокумарол, гепарин, клопидогрел, прасугрел, тикагрелор, ривароксабан, ацетилсалициловая кислота).
- Лекарства для лечения грибковых инфекций (например, кетоконазол, итраконазол), за исключением случаев, когда они применяются только на коже.
- Лекарства, используемые для лечения нерегулярного сердечного ритма (например, амиодарон, дронедарон, хинидин, верапамил).
Если вы используете лекарства, содержащие верапамил, ваш врач может указать вам использовать сниженную дозу дабигатрана этексилата в зависимости от заболевания, для которого он был назначен. См. также раздел 3.
- Лекарства для предотвращения отторжения трансплантированных органов (например, такролимус, циклоспорин).
- Комбинированный препарат глекапревира и пибрентасвира (антивирусное лекарство, используемое для лечения гепатита С).
- Противовоспалительные и обезболивающие лекарства (например, ацетилсалициловая кислота, ибупрофен, диклофенак).
- Травяной препарат для лечения депрессии.
- Антидепрессивные лекарства, называемые селективными ингибиторами обратного захвата серотонина или селективными ингибиторами обратного захвата серотонина и норэпинефрина.
- Рифампицин или кларитромицин (два антибиотика).
- Лекарства для лечения эпилепсии (например, карбамазепин, фенитоин).
Беременность и лактация
Неизвестны эффекты дабигатрана этексилата на беременность и плод. Не используйте это лекарство, если вы беременны, unless ваш врач указал, что это безопасно. Если вы способны к деторождению, избегайте беременности во время лечения дабигатраном этексилатом.
Не рекомендуется грудное вскармливание во время лечения дабигатраном этексилатом.
Вождение и использование машин
Дабигатран этексилат не имеет известных эффектов на способность управлять транспортными средствами и использовать машины.
3. Как принимать Дабигатран этексилат САГ
Дабигатран этексилат можно использовать у взрослых и детей от 8 лет, которые могут проглотить целые капсулы. Существуют другие лекарственные формы, подходящие для лечения детей младше 8 лет.
Следуйте точно инструкциям по применению этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом снова.
Принимайте дабигатран этексилат согласно рекомендациям для следующих ситуаций:
Профилактика цереброваскулярной или системной эмболии, вызванной образованием тромбов, развившейся после нерегулярного сердечного ритма, и лечение тромбов в венах ног и легких, включая профилактику повторного образования тромбов в венах ног и легких.
Рекомендуемая доза составляет 300 мг, вводимая в виде одной капсулы 150 мг дважды в день.
Если вам 80 лет или больше, рекомендуемая доза составляет 220 мг, вводимая в виде одной капсулы 110 мг дважды в день.
Если вы принимаете лекарства, содержащие верапамил, вам будет указано сниженная доза 220 мг, принимаемая в виде одной капсулы 110 мг дважды в день, поскольку ваш риск кровотечения может увеличиться.
Если у вас потенциально более высокий риск кровотечения, ваш врач может решить назначить вам дозу 220 мг, вводимую в виде одной капсулы 110 мг дважды в день.
Вы можете продолжать принимать это лекарство, если необходимо восстановить ваш нормальный сердечный ритм с помощью процедуры, называемой кардиоверсией или катетерной абляцией для фибрилляции предсердий. Принимайте дабигатран этексилат так, как указал ваш врач.
Если у вас установлен медицинский прибор (васкулярный стент) в кровеносном сосуде для поддержания его открытия в процедуре, называемой коронарной ангиопластикой с установкой стента, вы можете получить лечение дабигатраном этексилатом после того, как ваш врач решит, что достигнут нормальный контроль свертываемости крови. Принимайте дабигатран этексилат так, как указал ваш врач.
Лечение тромбов и профилактика повторного образования тромбов у детей
Дабигатран этексилат следует принимать дважды в день, одна доза утром и одна доза вечером, примерно в одно и то же время каждый день. Интервал между приемами должен быть как можно ближе к 12 часам.
Рекомендуемая доза зависит от веса и возраста. Ваш врач определит правильную дозу. Возможно, ваш врач изменит дозу во время лечения. Продолжайте использовать все другие лекарства, unless ваш врач указал вам прекратить использование какого-либо из них.
Таблица 1 показывает единичные и общедневные дозы дабигатрана этексилата в миллиграммах (мг). Дозы зависят от веса в килограммах (кг) и возраста пациента в годах.
Таблица 1: Таблица дозирования для дабигатрана этексилата
Комбинации вес/возраст | Единичная доза в мг | Общая дневная доза в мг | |
Вес в кг | Возраст в годах | ||
11 до менее 13 кг | 8 до менее 9 лет | 75 | 150 |
13 до менее 16 кг | 8 до менее 11 лет | 110 | 220 |
16 до менее 21 кг | 8 до менее 14 лет | 110 | 220 |
21 до менее 26 кг | 8 до менее 16 лет | 150 | 300 |
26 до менее 31 кг | 8 до менее 18 лет | 150 | 300 |
31 до менее 41 кг | 8 до менее 18 лет | 185 | 370 |
41 до менее 51 кг | 8 до менее 18 лет | 220 | 440 |
51 до менее 61 кг | 8 до менее 18 лет | 260 | 520 |
61 до менее 71 кг | 8 до менее 18 лет | 300 | 600 |
71 до менее 81 кг | 8 до менее 18 лет | 300 | 600 |
81 кг или более | 10 до менее 18 лет | 300 | 600 |
Единичные дозы, требующие комбинации более одной капсулы:
300 мг: две капсулы по 150 мг или четыре капсулы по 75 мг
260 мг: одна капсула 110 мг и одна капсула 150 мг или
одна капсула 110 мг и две капсулы по 75 мг
220 мг: две капсулы по 110 мг
185 мг: одна капсула 75 мг и одна капсула 110 мг
150 мг: одна капсула 150 мг или две капсулы по 75 мг
Как принимать Дабигатран этексилат САГ
Дабигатран этексилат можно принимать с или без пищи. Капсулу необходимо проглотить целиком с стаканом воды, чтобы обеспечить высвобождение в желудке. Не разрывайте, не жуйте и не открывайте капсулу, чтобы принять только ее содержимое, поскольку это может увеличить риск кровотечения.
Инструкции для открытия блистеров
Следующие изображения иллюстрируют, как извлечь капсулы дабигатрана этексилата из блистера:
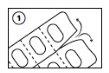
Отделите отдельный блистер от полоски блистеров через перфорированную линию.

Снимите заднюю пленку и извлеките капсулу
- Не продавливайте капсулы через пленку блистера.
- Не снимайте пленку блистера до тех пор, пока капсула не будет необходима.
Изменение антикоагулянтной терапии
Не меняйте свою антикоагулянтную терапию без конкретных указаний вашего врача.
Если вы приняли слишком много Дабигатрана этексилата САГ
Принятие слишком большого количества этого лекарства увеличивает риск кровотечения. Свяжитесь с вашим врачом немедленно, если вы приняли слишком много капсул. Существуют специальные варианты лечения.
В случае передозировки или случайного приема проконсультируйтесь с вашим врачом или фармацевтом или позвоните в Службу токсикологической информации, телефон: 91 562 04 20, указав лекарство и количество, принятое.
Если вы пропустили прием Дабигатрана этексилата САГ
Пропущенную дозу можно принять до 6 часов до следующей дозы.
Пропущенную дозу следует пропустить, если время до следующей дозы меньше 6 часов. Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы.
Если вы прекратите лечение Дабигатраном этексилатом САГ
Принимайте дабигатран этексилат точно так, как указано. Не прекращайте свое лечение этим лекарством без консультации с вашим врачом, поскольку риск развития тромба может быть выше, если вы прекратите лечение слишком рано.
Свяжитесь с вашим врачом, если у вас появляются проблемы с пищеварением после приема дабигатрана этексилата.
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди их испытывают.
Дабигатран этексилат действует на свертываемость крови; поэтому большинство побочных эффектов связаны с признаками, такими как синяки или кровотечения.
Могут возникать эпизоды сильного или тяжелого кровотечения, которые являются наиболее серьезными побочными эффектами и которые, независимо от их местоположения, могут привести к инвалидности, быть потенциально смертельными или даже вызывать смерть. В некоторых случаях эти кровотечения могут быть не очевидными.
Если вы испытываете любой эпизод кровотечения, который не останавливается самостоятельно, или если вы испытываете признаки чрезмерного кровотечения (исключительная слабость, усталость, бледность, головокружение, головная боль или необъяснимая отечность), немедленно обратитесь к врачу. Ваш врач может решить держать вас под пристальным наблюдением или изменить лекарство.
Сообщите врачу немедленно, если вы испытываете тяжелую аллергическую реакцию, которая вызывает трудности с дыханием или головокружение.
Возможные побочные эффекты перечислены ниже, сгруппированные по частоте их возникновения.
Профилактика цереброваскулярной или системной окклюзии, вызванной образованием тромбов, развившейся после аномального сердечного ритма
Частые (могут поражать до 1 из 10 человек):
- Кровотечение может быть из носа, в желудке или кишечнике, из половых органов или мочевыводящих путей (включая кровь в моче, которая окрашивает мочу в розовый или красный цвет), или под кожей
- Снижение количества красных кровяных телец в крови
- Боль в животе или боль в желудке
- Изжога
- Частые жидкие или мягкие стулы
- Дискомфорт
Редкие (могут поражать до 1 из 100 человек):
- Кровотечение
- Кровотечение может быть из геморроидальных узлов, прямой кишки или мозга
- Формирование гематом
- Кашель с кровью или мокротой с кровяными пятнами
- Снижение количества тромбоцитов в крови
- Снижение количества гемоглобина в крови (вещества, присутствующего в красных кровяных тельцах)
- Аллергическая реакция
- Внезапное изменение цвета и внешнего вида кожи
- Зуд
- Язва желудка или кишечника (включая язву пищевода)
- Воспаление пищевода и желудка
- Рефлюкс желудочного сока в пищевод
- Рвота
- Трудности с глотанием
- Аномалии в тестах функции печени
Очень редкие (могут поражать до 1 из 1000 человек):
- Кровотечение может быть в суставе, в месте хирургического разреза, в ране, в месте введения инъекции или в месте введения катетера в вену
- Тяжелая аллергическая реакция, которая вызывает трудности с дыханием или головокружение
- Тяжелая аллергическая реакция, которая вызывает отек лица или горла
- Сыпь на коже с темными красными бугорками, выступающими и зудящими, вызванными аллергической реакцией
- Снижение количества кровяных клеток
- Увеличение печеночных ферментов
- Желтушная окраска кожи или белого цвета глаз, вызванная проблемами с печенью или кровью
Частота неизвестна (частота не может быть оценена на основе доступных данных):
- Трудности с дыханием или свистящее дыхание
- Снижение количества или даже отсутствие лейкоцитов (которые помогают бороться с инфекциями)
- Потеря волос
В клиническом испытании частота сердечных приступов при приеме дабигатрана этексилата была численно выше, чем при варфарине. Общая частота была низкой.
Лечение тромбов в венах ног и легких, включая профилактику повторного образования тромбов в венах ног и/или легких
Частые (могут поражать до 1 из 10 человек):
- Кровотечение может быть из носа, в желудке или кишечнике, из прямой кишки, половых органов или мочевыводящих путей (включая кровь в моче, которая окрашивает мочу в розовый или красный цвет), или под кожей
- Изжога
Редкие (могут поражать до 1 из 100 человек):
- Кровотечение
- Кровотечение может быть в суставе или в ране
- Кровотечение может быть из геморроидальных узлов
- Снижение количества красных кровяных телец в крови
- Формирование гематом
- Кашель с кровью или мокротой с кровяными пятнами
- Аллергическая реакция
- Внезапное изменение цвета и внешнего вида кожи
- Зуд
- Язва желудка или кишечника (включая язву пищевода)
- Воспаление пищевода и желудка
- Рефлюкс желудочного сока в пищевод
- Дискомфорт
- Рвота
- Боль в животе или боль в желудке
- Частые жидкие или мягкие стулы
- Аномалии в тестах функции печени
- Увеличение печеночных ферментов
Очень редкие (могут поражать до 1 из 1000 человек):
- Кровотечение может быть в месте хирургического разреза, или в месте введения инъекции, или в месте введения катетера в вену или из мозга
- Снижение количества тромбоцитов в крови
- Тяжелая аллергическая реакция, которая вызывает трудности с дыханием или головокружение
- Тяжелая аллергическая реакция, которая вызывает отек лица или горла
- Сыпь на коже с темными красными бугорками, выступающими и зудящими, вызванными аллергической реакцией
- Трудности с глотанием
Частота неизвестна (частота не может быть оценена на основе доступных данных):
- Трудности с дыханием или свистящее дыхание
- Снижение количества гемоглобина в крови (вещества, присутствующего в красных кровяных тельцах)
- Снижение количества кровяных клеток
- Снижение количества или даже отсутствие лейкоцитов (которые помогают бороться с инфекциями)
- Желтушная окраска кожи или белого цвета глаз, вызванная проблемами с печенью или кровью
- Потеря волос
В программе клинических испытаний частота сердечных приступов при приеме дабигатрана этексилата была выше, чем при варфарине. Общая частота была низкой. Не было обнаружено никакого дисбаланса в частоте сердечных приступов у пациентов, леченных дабигатраном, по сравнению с пациентами, леченными плацебо.
Лечение тромбов и профилактика повторного образования тромбов у детей
Частые (могут поражать до 1 из 10 человек):
- Снижение количества красных кровяных телец в крови
- Снижение количества тромбоцитов в крови
- Сыпь на коже с темными красными бугорками, выступающими и зудящими, вызванными аллергической реакцией
- Внезапное изменение цвета и внешнего вида кожи
- Формирование гематом
- Носовое кровотечение
- Рефлюкс желудочного сока в пищевод
- Рвота
- Дискомфорт
- Частые жидкие или мягкие стулы
- Изжога
- Потеря волос
- Увеличение печеночных ферментов
Редкие (могут поражать до 1 из 100 человек):
- Снижение количества лейкоцитов (которые помогают бороться с инфекциями)
- Кровотечение может быть в желудке или кишечнике, из мозга, прямой кишки, половых органов или мочевыводящих путей (включая кровь в моче, которая окрашивает мочу в розовый или красный цвет), или под кожей
- Снижение количества гемоглобина в крови (вещества, присутствующего в красных кровяных тельцах)
- Снижение количества кровяных клеток
- Зуд
- Кашель с кровью или мокротой с кровяными пятнами
- Боль в животе или боль в желудке
- Воспаление пищевода и желудка
- Аллергическая реакция
- Трудности с глотанием
- Желтушная окраска кожи или белого цвета глаз, вызванная проблемами с печенью или кровью
Частота неизвестна (частота не может быть оценена на основе доступных данных):
- Отсутствие лейкоцитов (которые помогают бороться с инфекциями)
- Тяжелая аллергическая реакция, которая вызывает трудности с дыханием или головокружение
- Тяжелая аллергическая реакция, которая вызывает отек лица или горла
- Трудности с дыханием или свистящее дыхание
- Кровотечение
- Кровотечение может быть в суставе или в ране, в месте хирургического разреза, в месте введения инъекции или в месте введения катетера в вену
- Кровотечение может быть из геморроидальных узлов
- Язва желудка или кишечника (включая язву пищевода)
- Аномалии в тестах функции печени
Сообщение о побочных эффектах
Если вы испытываете любой тип побочного эффекта, обратитесь к врачу или фармацевту, даже если это возможные побочные эффекты, которые не указаны в этом описании. Вы также можете сообщить об этом напрямую через Систему испанской фармаковигиланции лекарственных средств для человека: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение Дабигатрана этексилата САГ
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке или блистере после «CAD». Дата истечения срока годности - последний день месяца, указанного.
Не храните при температуре выше 30°C
Храните в оригинальной упаковке для защиты от влаги.
Лекарства не должны выбрасываться в канализацию или мусор. Сдавайте упаковки и лекарства, которые вам больше не нужны, в пункт SIGRE аптеки. Спросите у фармацевта, как избавиться от упаковок и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Дабигатрана этексилата САГ
Активное вещество - дабигатран. Каждая твердая капсула содержит 172,95 мг дабигатрана этексилата (в форме мезилата), эквивалентного 150 мг дабигатрана этексилата.
- Другие компоненты - tartaric кислота, аравийская камедь, гипромеллоза 2910, диметикон 350, тальк и гидроксипропилцеллюлоза.
- Оболочка капсулы содержит каррагинан, хлорид калия, диоксид титана (E-171), гипромеллоза 2910 и индиго кармин (E-132).
Внешний вид продукта и содержание упаковки
Дабигатран этексилат САГ 150 мг - твердые капсулы с синей крышкой и белой или бледно-белой оболочкой, размер 0, заполненные гранулами светло-желтого или белого цвета.
Это лекарство выпускается в упаковках, содержащих:
Блистерные упаковки из алюминия/ОПА-АЛ-ПВХ по 10 x 1, 30 x 1 или 60 x 1 твердых капсул.
Множественная упаковка, содержащая 4 упаковки по 50 x 1 твердых капсул (200 твердых капсул), множественная упаковка, содержащая 3 упаковки по 60 x 1 твердых капсул (180 твердых капсул) или множественная упаковка, содержащая 2 упаковки по 50 x 1 твердых капсул (100 твердых капсул) с блистерными упаковками из алюминия/ОПА-АЛ-ПВХ.
Возможно, не все размеры упаковок будут выпускаться.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг:
Galenicum Health, S.L.U.
Сан Габриэль, 50
08950 Эсплугес де Льобрегат, Барселона, Испания.
Производитель: Февраль 2024
Galenicum Health, S.L.U.
Авда. Корнелья, 144
08950-Эсплугес де Льобрегат (Барселона)
Испания
или
SAG Manufacturing S.L.U
Крта. Н-I, Км 36
28750 Сан Агустин де Гуадаликс, Мадрид,
Испания
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Испания: Дабигатран этексилат САГ 150 мг твердые капсулы EFG
Мальта: Дабигатран этексилат САГ 150 твердые капсулы
Дата последнего обновления этого описания:Декабрь 2023
Подробная информация о этом лекарстве доступна на сайте Испанского агентства по лекарствам и медицинским изделиям (AEMPS) http://www.aemps.gob.es/

Сколько стоит ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ в Испании в 2026 году?
Средняя цена на ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ в январь, 2026 года составляет около 45.08 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках45.08 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫФорма выпуска: КАПСУЛА, 110 мгАктивное вещество: dabigatran etexilateПроизводитель: Adamed Laboratorios S.L.U.Требуется рецептФорма выпуска: КАПСУЛА, 150 мгАктивное вещество: dabigatran etexilateПроизводитель: Adamed Laboratorios S.L.U.Требуется рецептФорма выпуска: КАПСУЛА, 75 мгАктивное вещество: dabigatran etexilateПроизводитель: Adamed Laboratorios S.L.U.Требуется рецепт
Аналоги ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ в Польша
Аналог ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ в Украина
Врачи онлайн по ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ДАБИГАТРАН ЭТЕКСИЛАТ САГ 150 мг ТВЕРДЫЕ КАПСУЛЫ – по решению врача и с учетом местных правил.














