CRYSVITA 20 MG SOLUCION INYECTABLE

Cómo usar CRYSVITA 20 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
CRYSVITA 10mg solución inyectable
CRYSVITA 20mg solución inyectable
CRYSVITA 30mg solución inyectable
burosumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es CRYSVITA y para qué se utiliza
- Qué necesita saber antes de empezar a usar CRYSVITA
- Cómo usar CRYSVITA
- Posibles efectos adversos
- Conservación de CRYSVITA
- Contenido del envase e información adicional
1. Qué es CRYSVITA y para qué se utiliza
Qué es CRYSVITA
CRYSVITA contiene el principio activo burosumab. Se trata de un tipo de medicamento llamado anticuerpo monoclonal humano.
Para qué se utiliza CRYSVITA
CRYSVITA se utiliza para tratar la hipofosfatemia ligada al cromosoma X (XLH). Se utiliza en niños y adolescentes de 1 a 17 años, y en adultos.
CRYSVITA se utiliza para tratar la osteomalacia inducida por tumor (TIO) cuando el tumor que la causa no puede ser extirpado o localizado con éxito, en niños y adolescentes de 1 a 17 años y en adultos.
Qué es la hipofosfatemia ligada al cromosomaX(XLH)
La hipofosfatemia ligada al cromosoma X es una enfermedad genética.
- Las personas con XLH tienen niveles más altos de una hormona llamada factor de crecimiento de fibroblastos 23 (FGF23).
- El FGF23 reduce la cantidad de fosfato en la sangre.
- El nivel bajo de fosfato puede:
- dar lugar a que los huesos no se endurezcan correctamente y, en niños y adolescentes, a que no crezcan correctamente;
- producir dolor y rigidez en los huesos y las articulaciones.
Qué es la osteomalacia inducida por tumor (TIO)
- Las personas con TIO tienen niveles más altos de una hormona llamada FGF23 producida por ciertos tipos de tumores.
- El FGF23 reduce la cantidad de fosfato en la sangre.
- El nivel bajo de fosfato puede producir ablandamiento de los huesos, debilidad muscular, cansancio, dolor óseo y fracturas.
Cómo funciona CRYSVITA
CRYSVITA se fija al FGF23 en la sangre, impidiendo que el FGF23 actúe y aumente los niveles de fosfato en la sangre para que se puedan alcanzar niveles normales de fosfato.
2. Qué necesita saber antes de empezar a usar CRYSVITA
No use CRYSVITA
- si es alérgico a burosumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si toma suplementos de fosfato o ciertos suplementos de vitamina D (que contienen la denominada vitamina D activa [p. ej., calcitriol]);
- si ya tiene un nivel alto de fosfato en la sangre («hiperfosfatemia»);
- si tiene enfermedad renal grave o insuficiencia renal.
Reacciones alérgicas
Deje de usar CRYSVITA y póngase en contacto con su médico inmediatamente si presenta alguno de los siguientes efectos adversos, ya que podrían ser signos de una reacción alérgica:
- erupción y picor en todo el cuerpo;
- hinchazón grave de los párpados, la boca o los labios (angioedema);
- falta de aire;
- latido cardiaco rápido;
- sudoración.
No use CRYSVITA si alguno de los puntos anteriores se aplica en su caso. Si tiene dudas, consulte a su médico antes de empezar a usar CRYSVITA.
Advertencias y precauciones
Reacciones cutáneas
Puede presentar reacciones cutáneas en la zona de inyección, ver sección 4 para más información. Si estas reacciones son graves, informe a su médico.
Pruebas y comprobaciones
Su médico comprobará los niveles de fosfato y de calcio en la sangre y en la orina y podría realizarle también una ecografía renal durante el tratamiento para reducir el riesgo de hiperfosfatemia (demasiado fosfato en la sangre) y de mineralización ectópica (acumulación de calcio en tejidos como los riñones). También comprobará el nivel de la hormona paratiroidea en suero de vez en cuando.
Niños menores de 1año
No se debe administrar CRYSVITA a niños menores de 1 año, ya que no se ha estudiado la seguridad ni los efectos de este medicamento en este grupo de edad.
Otros medicamentos y CRYSVITA
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No use CRYSVITA e informe a su médico si está tomando:
- suplementos de fosfato;
- ciertos suplementos de vitamina D (que contienen la denominada vitamina D activa [p. ej., calcitriol]). Hay algunos suplementos de vitamina D que puede continuar usando o empezar a usar, y su médico le indicará cuáles son.
Consulte a su médico antes de empezar a usar CRYSVITA:
- si está tomando medicamentos que actúan en el cuerpo de la misma forma que el calcio («calcimiméticos»). Si se utilizan simultáneamente pueden reducir el nivel de calcio en la sangre;
- si es un paciente con TIO y está a punto de recibir tratamiento para el tumor subyacente (es decir, radioterapia o extirpación quirúrgica). En este caso, el tratamiento con CRYSVITA no se iniciará hasta después del tratamiento del tumor y si los niveles de fosfato sérico son bajos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. Esto se debe a que se desconoce si CRYSVITA afectará al bebé.
No se recomienda utilizar CRYSVITA durante el embarazo.
Si puede quedarse embarazada, debe utilizar un método anticonceptivo efectivo mientras utiliza CRYSVITA y durante como mínimo 14 semanas después de la última dosis. Debe hablar de esto con su médico.
Se desconoce si CRYSVITA pasa a la leche materna y no se puede excluir el riesgo en recién nacidos/niños. Debe hablar de esto con su médico.
Conducción, montar en bicicleta y uso de máquinas
Es posible que CRYSVITA cause mareos y afecte a la capacidad de montar en bicicleta, utilizar herramientas o máquinas o conducir. Si cree que le afecta, no monte en bicicleta, no utilice herramientas ni máquinas y no conduzca. Informe a su médico.
CRYSVITA contiene sorbitol
Este medicamento contiene 45,91 mg de sorbitol en cada vial equivalente a 45,91 mg/ml.
3. Cómo usar CRYSVITA
CRYSVITA se debe administrar mediante inyección por debajo de la piel (vía subcutánea) en la parte superior del brazo, abdomen, nalga o muslo. Un profesional sanitario le administrará este medicamento a usted o a su hijo. Alternativamente, su médico puede recomendarle que se lo inyecte usted mismo o que se lo inyecte a su hijo. Un profesional sanitario le mostrará cómo hacerlo. La primera autoinyección tras comenzar el tratamiento o tras cualquier cambio de dosis se debe realizar delante de él. Al final de este prospecto se incluye una sección detallada de «Instrucciones de uso». Siga siempre estas instrucciones cuidadosamente cuando se administre la inyección de CRYSVITA a usted mismo o a su hijo.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, enfermero o farmacéutico. En caso de duda, consulte de nuevo a su médico, enfermero o farmacéutico.
Qué cantidad de CRYSVITA necesitará
La dosis se determina en función del peso corporal. Su médico calculará la dosis correcta para usted.
Dosis para XLH y TIO
Será necesario inyectar su dosis de CRYSVITA:
- cada dos semanas en niños y adolescentes de 1 a 17 años;
- cada 4 semanas en adultos.
Su médico realizará unas comprobaciones para asegurar que recibe la dosis correcta y podrá cambiar la dosis o la frecuencia de administración en caso necesario.
Dosis máxima para pacientes con XLH
La dosis máxima que recibirá para el tratamiento de la XLH es 90 mg.
Dosis máxima para pacientes con TIO
La dosis máxima que recibirá para el tratamiento de la TIO:
- para niños de 1 a 12 años es 90 mg;
- para adolescentes de 13 a 17 años y para adultos es 180 mg.
Pacientes con TIO
Si es un paciente con TIO que requiere tratamiento del tumor subyacente (es decir, radioterapia o extirpación quirúrgica), su médico suspenderá el tratamiento con CRYSVITA. Una vez finalizado el tratamiento del tumor, su médico realizará controles de sus niveles de fosfato y reiniciará el tratamiento con CRYSVITA si los niveles de fosfato sérico son bajos.
Si le administran más CRYSVITA del que debe
Si cree que le han administrado una cantidad excesiva de CRYSVITA, informe a su médico inmediatamente.
Si se saltó una dosis de CRYSVITA
Si se salta una dosis, consulte a su médico inmediatamente. La dosis olvidada se debe administrar lo antes posible y su médico volverá a programar las dosis futuras como corresponden.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversosen niños y adolescentes con XLH
Muy frecuentes (pueden afectar a más de 1 de cada 10niñosyadolescentes)
- Absceso dental (infección)
- Tos
- Dolor de cabeza
- Mareo
- Vómitos
- Náuseas
- Diarrea
- Estreñimiento
- Caries dentales
- Erupción
- Dolor de músculos (mialgia) y de manos y pies
- Reacciones en el lugar de inyección, que pueden incluir:
- enrojecimiento o erupción
- dolor o picor
- hinchazón
- sangrado o hematomas
Estas reacciones en la zona de inyección normalmente son leves y ocurren en el plazo de 1 día tras la inyección y suelen mejorar en el plazo de 1 a 3 días.
- Fiebre
- Nivel bajo de vitamina D en la sangre
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Aumento del fosfato en la sangre
Efectos adversos en niños y adolescentes con TIO
No se conocen los efectos adversos en niños y adolescentes, ya que no se han realizado estudios clínicos.
Efectos adversos en adultos con XLH y TIO
Muy frecuentes (pueden afectar a más de 1 de cada 10adultos)
- Absceso dental (infección)
- Dolor de cabeza
- Mareo
- Síndrome de piernas inquietas (impulso irresistible de mover las piernas para detener las sensaciones incómodas, dolorosas o extrañas en las piernas, especialmente antes de dormir o durante la noche)
- Estreñimiento
- Dolor de espalda
- Espasmo muscular
- Reacciones en la zona donde se administra la inyección, que pueden incluir dolor o hinchazón
- Nivel bajo de vitamina D en la sangre
Frecuentes (pueden afectar hasta a 1 de cada 10adultos)
- Erupción
- Aumento del fosfato en la sangre
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de CRYSVITA
Mantener CRYSVITA fuera de la vista y del alcance de los niños.
No utilice CRYSVITA después de la fecha de caducidad que aparece en la caja y la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
Conservar el vial en la caja exterior para protegerlo de la luz.
No utilice CRYSVITA si contiene partículas visibles.
Los medicamentos no se deben tirar por los desagües ni a la basura. De esta forma, ayudará a proteger el medio ambiente.
Si se autoinyecta, consulte el paso 5 de las «Instrucciones de uso» que aparecen al final del prospecto para la eliminación de los medicamentos y materiales no utilizados. Si tiene alguna duda sobre cómo deshacerse de los medicamentos que ya no necesita, pregunte a su profesional sanitario o farmacéutico.
6. Contenido del envase e información adicional
Composición de CRYSVITA
El principio activo es burosumab. Cada vial contiene 10, 20 o 30 mg de burosumab.
Los demás componentes son L-histidina, D-sorbitol (E 420), polisorbato 80, L-metionina, ácido clorhídrico al 10 % y agua para preparaciones inyectables. (Ver «CRYSVITA contiene sorbitol» en la sección 2 para más información).
Aspecto del producto y contenido del envase
CRYSVITA se presenta como una solución inyectable transparente a ligeramente opalescente y de incolora a marrón-amarilla pálida, en un vial de vidrio pequeño. Cada envase contiene 1 vial.
Titular de la autorización de comercialización
Kyowa Kirin Holdings B.V.
Bloemlaan 2
2132NP Hoofddorp
Países Bajos
Responsable de la fabricación
allphamed PHARBIL Arzneimittel GmbH
Hildebrandstr. 10-12
37081 Göttingen
Alemania
Kyowa Kirin Holdings B.V.
Bloemlaan 2
2132NP Hoofddorp
Países Bajos
Fecha de la última revisión de este prospecto:junio 2025
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
INSTRUCCIONES DE USO
Lea atentamente estas instrucciones de uso antes de utilizar CRYSVITA:
- Inyéctese a usted mismo o a su hijo solo si se lo ha indicado su médico.
- Solo se debe inyectar después de haber recibido formación sobre la técnica de inyección. La primera autoinyección tras comenzar el tratamiento o tras cualquier cambio de dosis se debe realizar delante de un profesional sanitario.
- Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero (profesional sanitario). En caso de duda, consulte de nuevo a su profesional sanitario.
- Su médico le recetará la dosis correcta. Su dosis se mide en miligramos (mg). CRYSVITA está disponible en viales de tres concentraciones diferentes: 10 mg, 20 mg y 30 mg. Cada vial es de un solo uso. Utilice siempre un nuevo vial de CRYSVITA para cada inyección, ver el paso 5 sobre cómo desechar los viales usados y otros materiales.
- Su profesional sanitario le indicará la cantidad de CRYSVITA que se debe administrar a sí mismo o a su hijo. Usted o su hijo pueden recibir más de un vial para obtener la dosis correcta.
- Si su profesional sanitario le indica que es necesaria más de una inyección para administrar la dosis requerida, debe repetir los pasos 2 a 5 siguientes para cada inyección. Utilice nuevos materiales y un lugar diferente del cuerpo para cada inyección.
- Utilice únicamente la jeringa y las agujas proporcionadas o prescritas por su profesional sanitario para administrar la inyección.
- Utilice siempre la aguja grande para extraer el líquido y recuerde cambiar a la aguja pequeña para inyectar el líquido.
- El uso de una jeringa o aguja incorrecta puede llevar a un error en la dosis o hacer que la inyección sea más dolorosa.
- Cuando se administra CRYSVITA a un niño pequeño, puede ser útil que otra persona esté presente para calmarlo.
- No utilice CRYSVITA si es alérgico a alguno de los componentes de este medicamento. Deje de usar CRYSVITA si tiene alguna reacción alérgica durante o después de la inyección y póngase en contacto con su profesional sanitario inmediatamente. Ver la sección 2 del prospecto para obtener más información.
Paso 1. Reúna e inspeccione los materiales
Saque de la nevera los viales de CRYSVITA que necesite.
Compruebe la concentración en la etiqueta de cada vial.
Asegúrese de que tiene el número correcto de viales para que coincida con la dosis en mg aconsejada por su profesional sanitario.
Si no está seguro, pregunte a su profesional sanitario.
Deje que los viales se alcancen la temperatura ambiente durante 30 minutos. No caliente los viales de ninguna otra manera como, por ejemplo, con agua caliente o en un horno microondas. No exponga los viales a la luz solar directa.
Compruebe la fecha de caducidad (indicada después de CAD) en la etiqueta del vial. Inspeccione el líquido en el vial. No lo agite.
No utilice el vial si:
- está caducado;
- presenta un cambio de color, está turbio o contiene alguna partícula. El líquido de CRYSVITA debe ser de transparente a ligeramente opalescente y de incoloro a marrón-amarillo pálido.
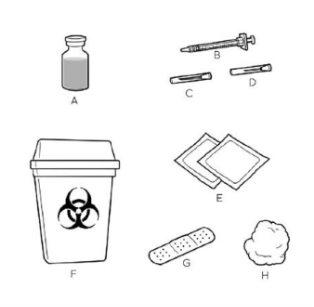
| Coloque todos los materiales que necesitará en una superficie limpia y plana. Para cada inyección necesitará:
Póngase en contacto con su profesional sanitario si no tiene estos materiales. Su profesional sanitario le explicará el uso de las diferentes agujas. |
La aguja grandese utiliza para extraer CRYSVITA del vial.
La aguja pequeñase utiliza para inyectar CRYSVITA.
Si no está seguro, pregunte a su profesional sanitario antes del uso.
No utilice ningún material al que le falten piezas o que esté dañado de alguna manera.
No retire los capuchones de las agujas hasta que esté listo para usarlas.
Lávese bien las manos con agua y jabón antes de pasar al paso 2.
Paso 2. Extraiga CRYSVITA y prepare la inyección
Retire la cápsula de cierre del vial para que quede el tapón de goma a la vista.
Limpie el tapón de goma con una toallita con alcohol y déjelo secar. No toque el tapón de goma después de limpiarlo.
| Seleccione la aguja grandey sáquela del envase estéril, pero no retire el capuchón que cubre la aguja. Para acoplar la aguja a la jeringa, sujete la aguja grandepor el capuchón protector en una mano y la jeringa por el cilindro en la otra. Dependiendo de los materiales que le hayan dado:
No toque la propia aguja ni el extremo de la jeringa donde se acopla la aguja. |
Una vez que la aguja esté firmemente acoplada, sujete la jeringa por el cilindro con la aguja apuntando
hacia arriba.
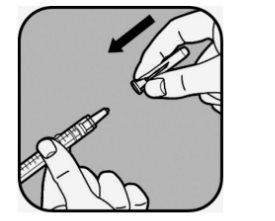
Retire el capuchón de la aguja tirando de él.
No tire el capuchón de la aguja.
No toque la aguja ni permita que esta entre en contacto con ninguna superficie una vez retirado el capuchón.
No utilice la jeringa si se le cae después de quitar el capuchón o si la aguja parece estar dañada.
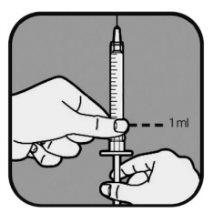
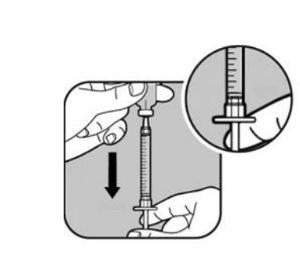
| Su profesional sanitario le indicará la cantidad de líquido que debe inyectarse. Normalmente será 1 ml por cada inyección. Su profesional sanitario le indicará qué marca debe utilizar si necesita inyectar menos de 1 ml. Utilice siempre la marca que corresponda a su dosis. Si no está seguro, pregunte a su profesional sanitario antes del uso. Tire del émbolo de la jeringa hasta que el extremo del émbolo se alinee con la marca correspondiente a su dosis. Así se llena la jeringa de aire. |
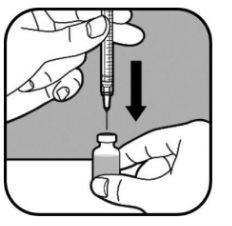
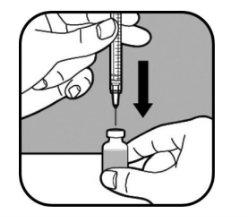
| Mantenga el vial en una superficie plana. Introduzca lentamente la aguja grande a través del tapón de goma y dentro del vial. No deje que la punta de la aguja toque el líquido del vial. Si la punta de la aguja toca el líquido, tire lentamente de la aguja hasta que deje de tocar el líquido. Empuje lentamente el émbolo en la jeringa. Así se empuja el aire de la jeringa hacia el vial. |
| Mantenga el vial en una superficie plana. Introduzca lentamente la aguja grande a través del tapón de goma y dentro del vial. No deje que la punta de la aguja toque el líquido del vial. Si la punta de la aguja toca el líquido, tire lentamente de la aguja hasta que deje de tocar el líquido. Empuje lentamente el émbolo en la jeringa. Así se empuja el aire de la jeringa hacia el vial. |
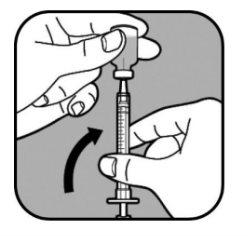
| Mantenga la aguja en el vial y póngalo boca abajo. Asegúrese de que la punta de la aguja esté en el fondo del líquido. |
| Tire lentamente del émbolo para llenar la jeringa hasta que el extremo del émbolo se alinee con la marca correspondiente a su dosis. Mantenga la punta de la aguja en el líquido en todo momento. |
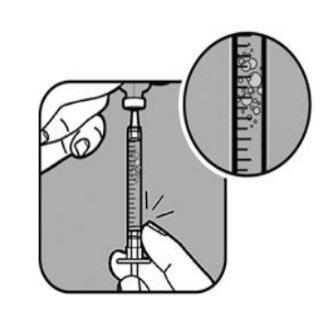
| Compruebe si hay burbujas de aire en el líquido de la jeringa. Si ve burbujas,
Compruebe de nuevo la dosis con las marcas de la jeringa. Si es necesario, extraiga un poco más de líquido para alinearlo con la marca correspondiente a su dosis. Compruebe de nuevo si hay burbujas y repita el proceso si es necesario. |
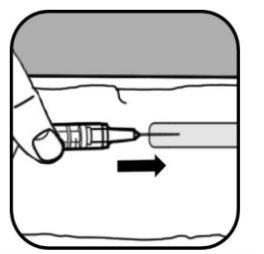
Cuando no haya burbujas en la jeringa, saque la jeringa y la aguja directamente del vial tirando hacia abajo.
| Retire la aguja grande de la jeringa.
|
Seleccione la aguja pequeñay retírela del envase estéril, pero no retire el capuchón que cubre la aguja.
Para acoplar la aguja en la jeringa, sujete la aguja pequeñapor el capuchón protector en una mano y la jeringa por el cilindro en la otra.
Dependiendo de los materiales que le hayan dado,
- tendrá que empujar la aguja hacia abajo y girar en el sentido de las agujas del reloj en la jeringa hasta que quede prieta;
- oempujar la aguja hacia abajo hasta que esté bien acoplada.
No toque la propia aguja ni el extremo de la jeringa donde se acopla la aguja.
Paso 3. Prepare el lugar de inyección
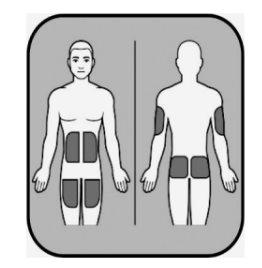
| La inyección debe administrase en la capa de grasa justo debajo de la piel. Tendrá que elegir el lugar de inyección. Si se está administrando la inyección a usted mismo, las zonas adecuadas son:
Si va a administrar la inyección a otra persona, las zonas adecuadas son:
No administre la inyección en:
|
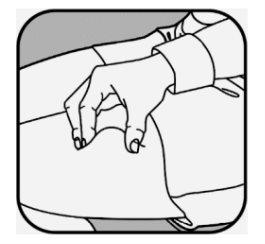
Si va a administrar más de una inyección, utilice un lugar diferente para cada inyección. Limpie cada lugar de inyección con una nueva toallita con alcohol y deje que la piel se seque.
CRYSVITA debe inyectarse en piel limpia y seca.
Paso 4. Administre la inyección de CRYSVITA
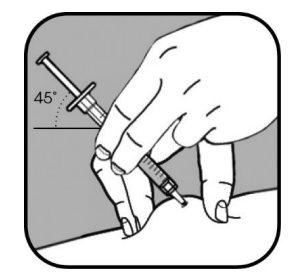
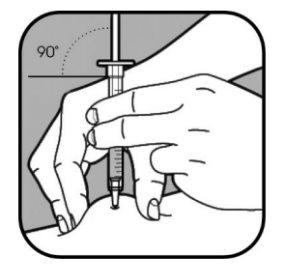
| Retire el capuchón de la aguja pequeña tirando de él. Pellizque la piel firmemente utilizando el pulgar y otros dedos, creando un área de unos 5 cm de ancho. Sujete la jeringa entre los dedos pulgar e índice de su mano dominante. La aguja se debe introducir en la piel en un ángulo de 45º o de 90º. Su profesional sanitario le indicará qué ángulo debe utilizar. |
|
|
Utilice un movimiento rápido como si fuera un dardo para introducir la aguja en la piel pellizcada.
No empuje el émbolo cuando introduzca la aguja.
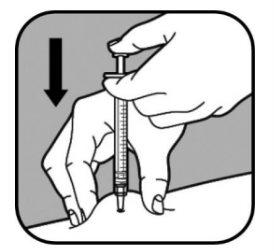
| Cuando la aguja esté introducida, no la mueva. Siga pellizcando la piel. Empuje lentamente el émbolo de la jeringa, durante un máximo de 30 segundos, hasta que la jeringa esté vacía. |
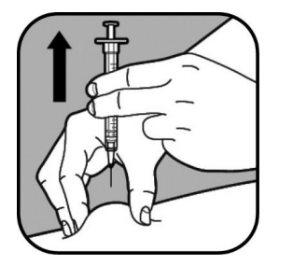
| Cuando haya administrado la dosis completa, retire la jeringa tirando suavemente de la misma hacia fuera. Suelte la piel pellizcada. Presione el lugar de inyección con un algodón o una gasa durante unos segundos para detener el sangrado. Ponga una tirita si es necesario. No frote el lugar de inyección. Para evitar cualquier herida, no vuelva a poner el capuchón en la aguja pequeña. Coloque la aguja sin tapar en el recipiente para objetos punzantes. |
Paso 5. Después de cada inyección
Deposite las agujas, los capuchones y las jeringas usados en el recipiente para objetos punzantes. Los viales se deben eliminar de acuerdo con las guías locales.
No tire las agujas ni las jeringas a la basura.
No guarde los viales con restos de medicamento para usarlos en el futuro ni se los dé a otras personas.
Cuando su recipiente para objetos punzantes esté casi lleno, deberá seguir las directrices locales para solicitar otro recipiente y eliminarlo correctamente.
Recordatorio:Si va a administrar más de una inyección, repita los pasos 2 a 5 para cada una de ellas.
Utilice materiales nuevos para cada inyección.
Anote la fecha de inyección y todas las zonas en las que se ha inyectado, de modo que utilice lugares diferentes para la siguiente inyección.
En el siguiente enlace hay un vídeo que muestra cómo preparar y administrar la inyección: www.myinject.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CRYSVITA 20 MG SOLUCION INYECTABLEForma farmacéutica: NULL, 10 mgPrincipio activo: BurosumabFabricante: Kyowa Kirin Holdings B.V.Requiere recetaForma farmacéutica: INYECTABLE, 10 mgPrincipio activo: BurosumabFabricante: Kyowa Kirin Holdings B.V.Requiere recetaForma farmacéutica: NULL, 20 mgPrincipio activo: BurosumabFabricante: Kyowa Kirin Holdings B.V.Requiere receta
Médicos online para CRYSVITA 20 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CRYSVITA 20 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes