CETRAFLUX 3 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS


Cómo usar CETRAFLUX 3 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
CETRAFLUX 3 mg/ml colirio en solución en envase unidosis
{Principio(s) activo(s)}
Lea todo el prospecto detenidamente antes de empezara usareste medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Contenido del prospecto
- Qué es Cetraflux y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cetraflux
- Cómo usar Cetraflux
- Posibles efectos adversos
- Conservación de Cetraflux
- Contenido del envase e información adicional
1. Qué es Cetraflux y para qué se utiliza
Cetraflux es un colirio.
Ciprofloxacino pertenece al grupo de antibióticos denominados quinolonas y a un subgrupo llamado fluoroquinolonas.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas como la gripe o el catarro. Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico. No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura. |
Cetraflux se utiliza para el tratamiento de infecciones causadas por bacterias en el ojo(s): conjuntivitis bacteriana purulenta, queratitis (inflamación bacteriana de la cornea), úlceras corneales y abscesos corneales.
2. Qué necesita saber antes de empezar a usar Cetraflux
No use Cetraflux
Si es alérgico a ciprofloxacino o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
- Sólo utilice este medicamento en sus ojo(s). No lo inyecte, no lo trague.
- Si experimenta reacciones alérgicas graves, erupción, sarpullido, picor o si tiene dificultad para respirar, interrumpa inmediatamente el tratamiento y consulte a su médico ya que esto puede ocurrir después de la primera dosis.
- Si los síntomas empeoran o reaparecen rápidamente, consulte a su médico. Con el uso de este medicamento puede volverse más sensible a otras infecciones.
- Si durante o poco después de usar gotas para los ojos, sufre dolor, hinchazón o inflamación de los tendones, interrumpa el tratamiento y consulte a su médico.
- Si utiliza lentes de contacto:
- Llevar lentes de contacto (duras o blandas) no está recomendado durante el tratamiento de una infección del ojo.
- Si está tomando cualquier otro medicamento vea sección Uso de Cetraflux con otros medicamentos.
Uso de Cetraflux con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Es preferible evitar este medicamento durante el embarazo a menos que el beneficio terapéutico supere el riesgo potencial para el feto.
Ciprofloxacino pasa a la leche materna. Si está dando el pecho a un bebé, consulte a su médico antes de utlizar Cetraflux.
Conducción y uso de máquinas
Si nota que su vista se altera de alguna manera tras el uso de Cetraflux no debe conducir o manejar maquinaria.
Información importante si utiliza lentes de contacto
No se aconseja utilizar lentes de contacto durante el tratamiento de una infección en el ojo, ya que su infección podría empeorar. No utilice el colirio si lleva las lentes de contacto puestas.
Espere al menos 15 minutos tras el uso del colirio antes de volver a colocarse las lentes de contacto.
Si tiene alguna duda más sobre el uso de Cetraflux, consulte a su médico o farmacéutico.
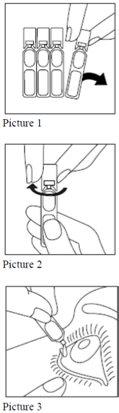
3. Cómo usar Cetraflux
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada será diferente según si usted está siendo tratado para abscesos corneales o para otras infecciones bacterianas en sus ojos.
Adultos, recién nacidos, niños y adolescentes
Abscesos corneales
El colirio debe ser administrado según los siguientes intervalos, incluso durante la noche.
- El primer día, instilar dos gotas en el ojo afectado cada 15 minutos, durante las primeras seis horas, y después dos gotas cada 30 minutos durante el resto del día.
- El segundo día instilar dos gotas en el ojo afectado cada hora.
- Desde el tercer día hasta el 14, instilar dos gotas en el ojo afectado cada 4 horas.
Si es necesario continuar el tratamiento con este medicamento después del día 14, su médico se lo indicará.
Otras infecciones del ojo:
- Durante los dos primeros días, instilar una o dos gotas en el ojo(s) afectado(s) cada 2 horas, durante el día, mientras está despierto.
- A partir del tercer día instilar una o dos gotas cada 4 horas durante el día.
Su médico le indicará la duración del tratamiento.
Es importante que continúe el tratamiento con Cetraflux hasta el final tal y como le ha indicado su médico.
Recomendaciones de uso
Utilice siempre Cetraflux siguiendo exactamente las instrucciones que le ha dado su médico.
Consulte con su médico o farmacéutico si tiene dudas.
|
|
Si una gota cae fuera del ojo, inténtelo de nuevo.
Si usa más Cetraflux colirio del que debe
Puede eliminarlo lavando los ojos con agua templada. No se aplique más gotas hasta que vuelva a tocarle.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si olvidó usar Cetraflux colirio
No utilice una dosis doble para compensar. Aplíquese una única dosis tan pronto como se dé cuenta, y luego vuelva a su régimen habitual. Si ya es casi la hora de la siguiente dosis, no se aplique la dosis olvidada y continúe con la siguiente dosis de su régimen habitual.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Cetraflux puede producir efectos adversos, aunque no todas las personas los sufran.
Puede experimentar algunos o todos los siguientes efectos adversos en su ojo:
Frecuentes(puedenafectarhasta1decada10personas): depósitos en la córnea, molestia ocular, hiperemia ocular (enrojecimiento ocular).
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): queratopatía (enfermedad no inflamatoria de la córnea), infiltrados corneales, tinción de la cornea, fotofobia (sensibilidad a la luz), agudeza visual disminuida (disminución de la visión), edema palpebral (párpado hinchado), visión borrosa, dolor ocular, ojo seco, hinchazón de los ojos , prurito ocular (picor en el ojo), sensación de cuerpo extraño en los ojos (sensación de tener algo en el ojo), lagrimeo incrementado (ojos llorosos), secreción ocular, costras en el margen del párpado, exfoliación del párpado, edema conjuntival, eritema del párpado (enrojecimiento de la parte interior párpado).
Raros (pueden afectar hasta 1 de cada 1.000 personas): toxicidad ocular, queratitis punteada (destrucción de las áreas exteriores de la córnea), queratitis (inflamación de la córnea), conjuntivitis, trastorno corneal, defecto del epitelio corneal, diplopía (visión doble), hipoestesia del ojo (disminución de la estimulación de la sensibilidad), astenopía (vista cansada), irritación ocular, inflamación ocular, hiperemia conjuntival. Si usted nota partículas blancas en los ojos, siga usando Cetraflux pero informe a su médico de inmediato.
Puede experimentar efectos en otras áreas de su cuerpo como:
Infecciones e infestaciones:
Raros (pueden afectar hasta 1 de cada 1.000 personas): orzuelo, rinitis (inflamación de la membrana mucosa dentro de la nariz).
Trastornos del sistema inmunológico:
Raros (pueden afectar hasta 1 de cada 1.000 personas): reacciones alérgicas como erupción cutánea, picor, hinchazón, dificultad para respirar).
Trastornos del sistema nervioso:
Frecuentes (pueden afectar hasta 1 de cada 10 personas): mal sabor de boca.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): dolor de cabeza.
Raros (pueden afectar hasta 1 de cada 1.000 personas): mareo.
Trastornos del oído:
Raros (pueden afectar hasta 1 de cada 1.000 personas): dolor de oído
Trastornos respiratorios:
Raros (pueden afectar hasta 1 de cada 1.000 personas): hipersecreción de los senos paranasales.
Trastornos gastrointestinales:
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): náuseas.
Raros (pueden afectar hasta 1 de cada 1.000 personas): diarrea, dolor abdominal.
Trastornos de la piel:
Raros (pueden afectar hasta 1 de cada 1.000 personas): dermatitis (inflamación de la piel).
Trastornos generales:
Raros (pueden afectar hasta 1 de cada 1.000 personas): intolerancia a medicamentos.
Exploraciones complementarias:
Raros (pueden afectar hasta 1 de cada 1.000 personas): resultados de test de laboratorio anormales.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Si experimenta una reacción alérgica, no utilice más Cetraflux y consulte a su médico.
5. Conservación de Cetraflux
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30°C. Conservar la ampolla en el embalaje exterior para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad (EXP) que está impresa en la caja. La fecha de caducidad es el último día del mes que se indica. La caducidad una vez abierto el sobre es de 90 días.
Caducidad tras abrir la ampolla de colirio: el contenido de la ampolla debe utilizarse inmediatamente una vez abierto. Cualquier cantidad que sobre debe desecharse.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cetraflux
- El principio activo es ciprofloxacino. Cada ml de solución contiene 3 mg de ciprofloxacino (como hidrocloruro).
- Los demás componentes son manitol, edetato de disodio, ácido acético glacial, acetato de sodio trihidrato, ácido clorhídirico y agua purificada.
Aspecto del producto y contenido del envase
Cetraflux es un colirio en solución acuosa estéril y sin conservante, transparente, incoloro o ligeramente amarillento que se presenta en formatos de 40 ampollas. Cada ampolla proporciona 0,25 ml. Las ampollas deben conservarse dentro de la bolsa para su protección.
Titular de la autorización de comercialización y responsable de la fabricación
Laboratorios Salvat, S.A.
Gall 30 - 36
08950 Esplugues de Llobregat (Barcelona)
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Cetraflux 3mg/ml Colírio, solução | Portugal Laboratorios Salvat, S.A. Gall 30 - 36 08950 Esplugues de Llobregat (Barcelona) |
Cetraflux 3mg/ml collirio, soluzione | Italia Laboratorios Salvat, S.A. Gall 30 - 36 08950 Esplugues de Llobregat (Barcelona) |
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Fecha de la última revisión de este prospecto:
- País de registro
- Precio medio en farmacia4.64 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CETRAFLUX 3 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: POMADA OFTALMICA, 3,5 gramosPrincipio activo: CiprofloxacinoFabricante: Ntc S.R.L.Requiere recetaForma farmacéutica: COLIRIO, 3,5 mg ciprofloxacino hidrocloruroPrincipio activo: CiprofloxacinoFabricante: Ntc S.R.L.Requiere recetaForma farmacéutica: COLIRIO, 5 mg/mlPrincipio activo: moxifloxacinaFabricante: Tiedra Farmaceutica S.L.Requiere receta
Médicos online para CETRAFLUX 3 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CETRAFLUX 3 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes