
BUDESONIDA/FORMOTEROL CIPLA 160 MICROGRAMOS/4,5 MICROGRAMOS/INHALACION POLVO PARA INHALACION (UNIDOSIS)


Cómo usar BUDESONIDA/FORMOTEROL CIPLA 160 MICROGRAMOS/4,5 MICROGRAMOS/INHALACION POLVO PARA INHALACION (UNIDOSIS)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Budesonida/Formoterol Cipla 160 microgramos/4,5 microgramos/inhalación, polvo para inhalación (unidosis)
Budesonida/formoterol fumarato dihidrato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Budesonida/Formoterol Cipla y para qué se utiliza
- Qué necesita saber antes de empezar a usar Budesonida/Formoterol Cipla
- Cómo usar Budesonida/Formoterol Cipla
- Posibles efectos adversos
- Conservación de Budesonida/Formoterol Cipla
- Contenido del envase e información adicional
1. Qué es Budesonida/Formoterol Cipla y para qué se utiliza
Budesonida/Formoterol Cipla es un inhalador que se utiliza para el tratamiento de:
- asma en adultos y adolescentes entre 12 y 17 años de edad.
- También se utiliza para el tratamiento sintomático de la Enfermedad Pulmonar Obstructiva Crónica (EPOC) en adultos mayores de 18 años .
Contiene dos medicamentos diferentes: budesonida y formoterol fumarato dihidrato.
- Budesonida pertenece a un grupo de medicamentos denominados “corticosteroides”, y actúa reduciendo y previniendo la inflamación de sus pulmones.
- Formoterol fumarato dihidrato pertenece a un grupo de medicamentos denominados “agonistas beta2 adrenérgicos de acción larga” o “broncodilatadores”, y actúa relajando los músculos de las vías respiratorias, lo que le ayuda a respirar más fácilmente.
Asma
Budesonida/Formoterol Cipla puede recetarse para el asma de dos formas diferentes:
- A algunos pacientes se les recetan dos inhaladores diferentes: Budesonida/Formoterol Cipla y otro inhalador por separado “para el alivio de los síntomas”.
- Los pacientes utilizan Budesonida/Formoterol Cipla a diario, lo que ayuda a prevenir la aparición de los síntomas del asma.
- Los pacientes utilizan su “inhalador de alivio” cuando presentan síntomas de asma, para facilitar la respiración.
- A algunos pacientes se les receta Budesonida/Formoterol Cipla como único inhalador para el asma.
- Los pacientes utilizan Budesonida/Formoterol Cipla a diario, lo que ayuda a prevenir la aparición de los síntomas del asma.
- Los pacientes también utilizan Budesonida/Formoterol Cipla cuando necesitan dosis adicionales para el alivio de los síntomas del asma, facilitando la respiración, y no necesitan otro inhalador por separado para este fin.
Enfermedad pulmonar obstructiva crónica (EPOC)
Budesonida/Formoterol Cipla también se puede utilizar para el tratamiento de los síntomas de EPOC en adultos. La EPOC es una enfermedad crónica caracterizada por provocar dificultades respiratorias continuas de las vías respiratorias pulmonares,acompañadas de tos y flemas. A menudo es causada por el tabaco.
2. Qué necesita saber antes de empezar a usar Budesonida/Formoterol Cipla
No use Budesonida/Formoterol Cipla:
- si es alérgico a budesonida, formoterol o a alguno de los demás componente de este medicamento (incluido en la sección 6), que es lactosa (que contiene pequeñas cantidades de proteínas de la leche).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Budesonida/Formoterol Cipla si:
- tiene diabetes,
- presenta alguna infección pulmonar,
- tiene la tensión arterial alta, o alguna vez ha tenido alguna enfermedad de corazón (incluyendo latidos irregulares, pulso acelerado, estrechamiento de las arterias o insuficiencia cardiaca),
- tiene problemas de tiroides o de las glándulas suprarrenales,
- presenta niveles bajos de potasio en la sangre,
- tiene problemas graves de hígado.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Uso en deportistas
Este medicamento contiene formoterol que puede producir un resultado positivo en las pruebas de control de dopaje.
Uso de Budesonida/Formoterol Cipla con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
En particular, informe a su médico o farmacéutico si está usando cualquiera de los siguientes medicamentos:
- medicamentos beta-bloqueantes (como atenolol o propranolol para la tensión arterial alta), incluyendo colirios (como timolol para el glaucoma),
- medicamentos para tratar el ritmo cardiaco acelerado o irregular (como quinidina, disopiramida, procainamida),
- Medicamentos para tratar alergias, también llamados antihistamínicos, como terfenadina.
- Oxitocina, un medicamento para facilitar el parto.
- Procarbazina, un medicamento para tratar el cáncer.
- medicamentos como digoxina, utilizados habitualmente para tratar la insuficiencia cardiaca,
- diuréticos (como furosemida), utilizados para el tratamiento de la tensión arterial alta,
- Corticosteroides (como prednisolona). Estos se usan para tratar la inflamación o prevenir el rechazo de trasplantes de órganos.
- Medicamentos de xantina (como teofilina o aminofilina). Estos se usan a menudo para tratar el asma.otros broncodilatadores (como salbutamol),
- Otros medicamentos para ensanchar las vías respiratorias, también llamados broncodilatadores (como el salbutamol).
- Medicamentos para tratar la depresión, también llamados antidepresivos tricíclicos (como la amitriptilina) y la antidepresiva nefazodona.
- Medicamentos para tratar trastornos mentales, náuseas o vómitos, llamados medicamentos de fenotiazina (como la clorpromazina y la proclorperazina).
- Medicamentos para tratar infecciones fúngicas (como ketoconazol, itraconazol, voriconazol, posaconazol) e infecciones bacterianas (como claritromicina y telitromicina, furazolidona).
- Medicamentos para la enfermedad de Parkinson (como la levodopa).
- Medicamentos para problemas de tiroides (como levotiroxina).
- Ritonavir, cobicistat (medicamentos para tratar infecciones por VIH). Los efectos de Budesonida/Formoterol Cipla pueden aumentar y es posible que su médico desee controlarlo cuidadosamente.
Si se encuentra en cualquiera de estas situaciones, o si no está seguro, pregunte a su médico o farmacéutico antes de utilizar Budesonida/Formoterol Cipla.
Informe también a su médico o farmacéutico si se va a someter a anestesia general por una operación quirúrgica o por tratamiento dental.
Embarazo, lactancia y fertilidad
- Si está embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar Budesonida/Formoterol Cipla a menos que su médico se lo indique.
- En caso de quedarse embarazada durante el tratamiento con Budesonida/Formoterol Cipla, no deje de utilizarlo y consulte con su médico inmediatamente.
- Si se encuentra en periodo de lactancia, consulte con su médico antes de utilizar Budesonida/Formoterol Cipla.
Conducción y uso de máquinas
La influencia de Budesonida/Formoterol Cipla sobre la capacidad de conducir y de manejar maquinaria es nula o insignificante.
Budesonida/Formoterol Cipla contiene lactosa
Budesonida/Formoterol Cipla contiene lactosa, que es un tipo de azúcar. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de usar este medicamento. La cantidad de lactosa en este medicamento normalmente no suele causar problemas en personas intolerantes a la lactosa.
El excipiente lactosa contiene pequeñas cantidades de proteínas de la leche que pueden provocar reacciones alérgicas en pacientes con alergia a la proteína de la leche de vaca.
3. Cómo usar Budesonida/Formoterol Cipla
- Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- Es importante utilizar Budesonida/Formoterol Cipla a diario, aunque no presente síntomas de asma o EPOC en ese momento.
- Si está utilizando Budesonida/Formoterol Cipla para el asma, su médico querrá comprobar regularmente cómo van sus síntomas.
Si ha estado tomando comprimidos de corticoides para el asma o la EPOC, su médico puede reducir el número de comprimidos que toma, una vez que comience a usar Budesonida/Formoterol Cipla. Si ha estado tomando comprimidos de corticoides orales durante mucho tiempo, es posible que su médico le pida que se haga análisis de sangre de vez en cuando. Puede sentir malestar general cuando se le reduzca el número de corticoides orales, aunque sus síntomas pulmonares puedan estar mejorando. Es posible que experimente síntomas como congestión o goteo nasal, debilidad o dolor en las articulaciones o en los músculos y erupción cutánea (urticaria). Si le preocupa alguno de estos síntomas, o si presenta síntomas tales como dolor de cabeza, cansancio, náuseas o vómitos, póngase en contacto con su médico inmediatamente. Es posible que deba tomar otro medicamento si desarrolla síntomas alérgicos o artríticos. Debe consultar con su médico si le preocupa como debe seguir usando Budesonida/Formoterol Cipla.
Su médico puede considerar añadir comprimidos de corticoides para su tratamiento habitual durante los períodos de estrés (por ejemplo, cuando tiene una infección en el pecho o antes de una operación).
Información importante sobre los síntomas de asma o EPOC
Si mientras utiliza Budesonida/Formoterol Cipla siente dificultad para respirar o emite “pitos” con la respiración, debe seguir utilizando Budesonida/Formoterol Cipla pero contacte con su médico lo antes posible, ya que podría necesitar un tratamiento adicional.
Contacte inmediatamente con su médico si:
- Su respiración está empeorando o a menudo se despierta por la noche con síntomas de asma.
- Empieza a sentir opresión en el pecho por la mañana, o la opresión en el pecho se prolonga más de lo normal.
- Estos signos pueden indicar que su asma o EPOC no están adecuadamente controlados y usted puede necesitar inmediatamente un tratamiento diferente o adicional.
Asma
Budesonida/Formoterol Cipla se puede recetar para el asma de dos formas diferentes, por lo que la cantidad a utilizar y cuándo utilizarla depende de cómo se lo haya indicado el médico:
- Si su médico le ha recetado Budesonida/Formoterol Cipla y otro inhalador de alivio de los síntomas por separado, lea el apartado “a) Utilización de Budesonida/Formoterol Cipla y otro inhalador por separado para el alivio de los síntomas”.
- Si su médico le ha recetado Budesonida/Formoterol Cipla como único inhalador, lea el apartado “b) Utilización de Budesonida/Formoterol Cipla como único inhalador para el asma”.
- Utilización de Budesonida/Formoterol Ciplay otro inhalador por separado para el alivio de los síntomas
Utilice Budesonida/Formoterol Cipla a diario,ya que ayuda a prevenir la aparición de los síntomas del asma.
Adultos (a partir de 18 años)
- La dosis habitual es 1 o 2 inhalaciones, dos veces al día.
- Su médico le aumentar hasta un máximo de 4 inhalaciones, dos veces al día.
- Si sus síntomas están bien controlados su médico le puede pedir que use la medicación una vez al día.
Adolescentes (de 12 a 17 años de edad)
- La dosis habitual es 1 o 2 inhalaciones, dos veces al día.
- Si sus síntomas están bien controlados su médico le puede pedir que use la medicación una vez al día.
No está recomendado el uso de Budesonida/Formoterol Cipla en niños menores de 12 años de edad.
Su médico (o enfermero) le ayudará a tratar el asma. Ellos ajustarán la dosis de este medicamento a la dosis más baja que controle su asma. Sin embargo, no ajuste la dosis sin hablar primero con su médico o enfermero.
Utilice su otro “inhalador para el alivio de los síntomas” para tratar síntomas de asma.Mantenga siempre consigo este “inhalador de alivio” para poder usarlo cuando lo necesite. No utilice Budesonida/Formoterol Cipla para tratar los síntomas del asma, sino su inhalador de alivio de los síntomas.
- Utilización de Budesonida/Formoterol Ciplacomo único inhalador para el asma
Utilice Budesonida/Formoterol Cipla de esta manera únicamente si su médico se lo ha indicado y si es mayor de 12 años.
Utilice Budesonida/Formoterol Ciplaya que ayuda a prevenir la aparición de los síntomas del asma. Puede utilizar:
- 1 inhalación por la mañana y 1 inhalación por la noche
o
- 2 inhalaciones por la mañana
o
- 2 inhalaciones por la noche.
Su médico puede aumentarle la dosis a 2 inhalaciones, dos veces al día.
Utilice también Budesonida/Formoterol Ciplacomo “inhalador de alivio” para tratar los síntomas del asma cuando éstos aparezcan.
- Si tiene síntomas de asma, realice 1 inhalación y espere unos minutos.
- Si no se encuentra mejor, realice otra inhalación.
- No realice más de 6 inhalaciones de una sola vez.
Mantenga siempre su inhalador de Budesonida/Formoterol Cipla consigo para poder utilizarlo cuando lo necesite.
Normalmente no se requiere una dosis total mayor de 8 inhalaciones al día. Sin embargo, su médico le puede permitir utilizar hasta 12 inhalaciones al día durante un periodo de tiempo limitado.
Si habitualmente necesita utilizar 8 o más inhalaciones al día, acuda a su médico o enfermero, ya que puede que necesiten cambiarle el tratamiento.
No realice nunca más de 12 inhalaciones en total en 24 horas.
Si está practicando ejercicio físico y nota síntomas de asma, utilice Budesonida/Formoterol Cipla tal como se ha descrito antes, no lo utilice justo antes del ejercicio para prevenir la aparición de los síntomas.
Enfermedad pulmonar obstructiva crónica (EPOC)
- Utilización sólo en adultos (mayores de 18 años).
- La dosis habitual es 2 inhalaciones, dos veces al día.
Su médico también le puede prescribir otros medicamentos broncodilatadores, por ejemplo anticolinérgicos (como el bromuro de ipratropio o de tiotropio) para su enfermedad de EPOC.
Instrucciones de uso:
Su médico, enfermero o farmacéutico le deben enseñar cómo utilizar el inhalador y revisar periódicamente que lo utilizan correctamente.
El inhalador contiene 60 dosis de medicamento en polvo en una tira de aluminio enrollada. Tiene un contador de dosis que le indica cuántas dosis restantes quedan en orden decreciente de 60 a 0. Cuando se alcanzan las 10 últimas dosis, los números aparecen sobre un fondo rojo.
El inhalador no es recargable, éste debe ser desechado cuando esté vació y reemplacelo por uno nuevo.
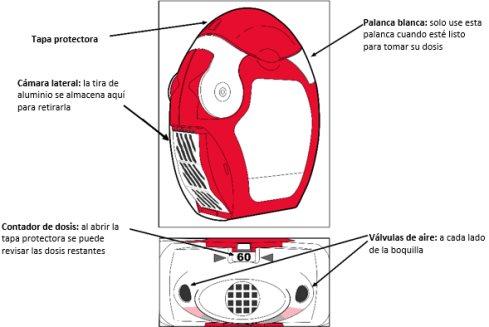
Antes de utilizar el inhalador:
- Debe abrir la cámara lateral transparente del inhalador.
- La tira de aluminio se debe cortar de la cámara lateral tirando cuidadosamente de la tira contra los “dientes” de la cámara lateral tal y como se muestra a continuación. No se debe tirar con fuerzade la tira o arrancarla.
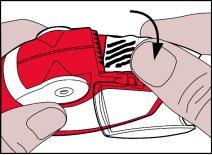
- Cierre la tapa de la cámara lateral y deseche la tira usada.
Importante:
A medida que el inhalador se va utilizando la cámara lateral se va llenando gradualmente con la tira de aluminio usada. Las tiras de aluminio con líneas negras no contienen medicamento. Eventualmente, las secciones numeradas de la tira aparecerán en la cámara lateral.
Nunca debe haber más de 2 secciones de lámina de aluminioen la cámara lateral ya que esto puede causar que el inhalador se atasque. La tira sobrante se debe cortar con cuidado y dejarla en un lugar seguro.
Utilización del inhalador:
Coja el inhalador con las manos, tal y como se muestra en las imágenes.
- Apertura
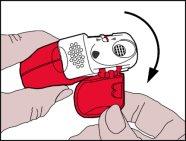
- La tapaprotectora se debe abrir hacia abajopara mostrar la boquilla.
- Se debe revisar el contador de dosis para ver cuántas dosis quedan.
- Preparación de la dosis
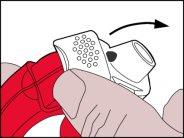
- Se debe subir hacia arribael borde de la palanca blanca. Asegúrese de que la cámara lateral está cerrada.
Recuerde:Tan sólo se debe manipular la palanca blanca cuando el paciente este listo para inhalar su dosis de medicación. Si el paciente juega con la palanca blanca puede desperdiciar dosis.
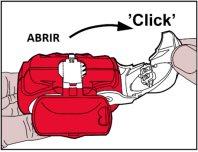
- Apertura:La palanca blanca se debe abrir completamentehasta dónde este su tope y hasta que haga un “click”. Esta acción mueve una nueva dosis a su posición con su número correspondiente arriba en el contador.
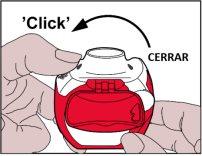
- Cierre:A continuación, la palanca blanca se debe cerrar completamentehasta que haga “click”de nuevo en su posición original. De esta forma el inhalador está listo para su uso inmediato.
- Inhalación de la dosis
- Con la boquilla del inhalador lejos de la boca, el paciente debe exhalar todo lo que pueda hasta que se sienta cómodo. Nunca debe exhalar directamente sobreel inhalador ya que esto puede afectar a la dosis.
- El inhalador se debe sujetar con la tapa protectora mirando hacia abajo.
- Se debe cerrar los labios firmemente alrededor de la boquilla.
- El paciente debe inhalartan fuerte y profundamentecomo le sea posible a través del inhalador, sin respirar a través de la nariz.
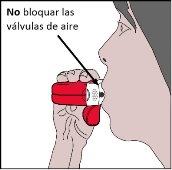
- A continuación, se debe retirar el inhalador de la boca y mantener la respiración durante 5 a 10 segundoso tanto como le sea posible sin que le cause malestar.
- A continuación, el paciente debe comenzar a respirar lentamente, pero fuera del inhalador.
- Se debe cerrar la tapa protectora de la boquilla.
- Se debe enjuagar la boca con agua, que se debe escupir a continuación. Esto le puede ayudar a prevenir infecciones fúngicas en la boca y evitar la ronquera.
Limpieza
- Si fuese necesario, la parte exterior de la boquilla se puede limpiar con un pañuelo seco.
- ¡Nunca separe las partes del inhalador para limpiarlas ni para cualquier otro propósito!
- ¡Las partes del inhalador no se pueden limpiar con agua o con paños húmedos ya que la humedad puede afectar a la dosis!
- ¡Nunca inserte imperdibles o cualquier otro objeto punzante dentro de la boquilla, o en cualquier otra parte, ya que esto puede dañar su inhalador!
Si usa más Budesonida/Formoterol Cipladel que debe
Es importante que emplee la dosis que se indica en el prospecto o la que su médico le ha prescrito. No debe aumentar su dosis sin consultar con su médico.
Los síntomas y signos más habituales que se pueden producir si usa más Budesonida/Formoterol Cipla del que debe son temblores, dolor de cabeza y latidos rápidos e irregulares del corazón.
Si ha tomado más Budesonida/Formoterol Cipla del que debe, consulte inmediatamente a su médico, a su enfermero o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Budesonida/Formoterol Cipla
- Si usted olvida alguna de las dosis, tómela tan pronto como se acuerde. Sin embargo, si es casi la hora de su siguiente dosis, no se preocupe por la dosis olvidada.
- Nouse una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si le ocurren cualquiera de las siguientes situaciones, deje de utilizar Budesonida/Formoterol Cipla y consulte inmediatamente con su médico:
- Se le hincha la cara, particularmente alrededor de la boca (lengua y/o garganta y/o dificultad para tragar) o urticaria junto con dificultades para respirar (angioedema) y/o sensación repentina de desfallecimiento, lo que indica que puede estar sufriendo una reacción alérgica. Esto ocurre raramente, ( puede afectar hasta 1 de cada 1.000 pacientes).
- Tiene “pitos” agudos o dificultad para respirar inmediatamente después de usar su inhalador. Si le sucede cualquiera de estos síntomas, deje de utilizar inmediatamente Budesonida/Formoterol Cipla y use su “inhalador de alivio”. Contacte inmediatamente con su médico ya que puede necesitar cambiar su tratamiento.Esto ocurre muy raramente, ( puede afectar hasta 1 de cada 10.000 pacientes).
Otros posibles efectos adversos:
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
- Palpitaciones (nota los latidos del corazón), temblores. Cuando aparecen estos efectos, suelen ser leves y desaparecen al continuar utilizando su Budesonida/Formoterol Cipla.
- Muguet (infección por hongos) en la boca. Este efecto es menos probable si se aclara la boca con agua después de utilizar su Budesonida/Formoterol Cipla.
- Irritación leve de garganta, tos, ronquera.
- Dolor de cabeza.
- Neumonía (infección en los pulmones) en pacientes con EPOC.
Informe a su médico si usted tiene cualquiera de los siguientes síntomas mientras inhala Budesonida/Formoterol Cipla, podrían ser síntomas de una infección pulmonar:
- fiebre o escalofríos,
- aumento de la producción de moco, cambio en el color del moco,
- aumento de la tos o aumento de dificultades para respirar.
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes)
- Agitación, inquietud, nerviosismo.
- Dificultad para dormir.
- Mareos.
- Visión borrosa.
- Náuseas (malestar).
- Ritmo cardiaco acelerado.
- Hematomas en la piel.
- Calambres musculares.
- Agresividad.
- Ansiedad
Raros(pueden afectar hasta 1 de cada 1.000 pacientes)
- Erupción, picor.
- Broncoespasmo (contracción de los músculos de las vías respiratorias, lo que provoca “pitos”). Si los pitidos le ocurre repentinamente justo después de utilizar Budesonida/Formoterol Cipla, deje de utilizarlo y consulte a su médico inmediatamente.
- Niveles bajos de potasio en sangre.
- Latido cardiaco irregular.
Muy raros(pueden afectar hasta 1 de cada 10.000 pacientes)
- Depresión.
- Cambios en el comportamiento, especialmente en niños.
- Dolor u opresión en el pecho (angina de pecho).
- Aumento en la cantidad de azúcar (glucosa) en sangre.
- Alteraciones del gusto, como sabor de boca desagradable.
- Variaciones en la tensión arterial.
- Aumento de peso, cara de luna llena, debilidad, obesidad abdominal (Sindrome de Cushing).
Los corticoides inhalados pueden afectar a la producción normal de hormonas esteroides en el organismo, especialmente si se utilizan dosis elevadas durante mucho tiempo. Estos efectos incluyen:
- cambios en la densidad mineral ósea (disminución de los huesos),
- cataratas (pérdida de transparencia del cristalino en el ojo),
- glaucoma (aumento de la presión ocular),
- retraso del crecimiento en niños y adolescentes,
- efectos sobre las glándulas suprarrenales (glándulas de pequeño tamaño situadas junto a los riñones),
- características cushingoides,
- también puede ocurrir un aumento en la susceptibilidad de sufrir infecciones y el deterioro de la capacidad para adaptarse al estres.
Estos efectos son mucho menos probables con los corticoides inhalados que con los comprimidos de corticoides.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Budesonida/Formoterol Cipla
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30 ºC
No utilice este medicamento después de la fecha de caducidad que aparece en envase o etiqueta del inhalador después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deBudesonida/Formoterol Cipla
- Los principios activos son budesonida y formoterol fumarato dihidrato. Cada dosisinhalada contiene 160 microgramos de budesonida y 4,5 microgramos de formoterol fumarato dihidrato esto corresponde a una dosis medida (unidosis contenida en el blíster) de 194,7 microgramos de budesonida y 6,1 microgramos de formoterol fumarato dihidrato.
- El otro componente es lactosa monohidrato (que contiene proteínas de la leche).
Aspecto del producto y contenido del envase
Budesonida/Formoterol Cipla es un inhalador de plástico de color rojo/blanco que contiene su medicamento. Cada inhalador contiene un blíster de OPA/Al/PVC-Al con 60 dosis pre-medidas de polvo mezclado. El polvo inhalado es de blanco a blanquecino o ligeramente amarillento sin aglomerados.
Budesonida/Formoterol Cipla está disponible en envases de 1, 2, 6 inhaladores, cada uno contiene 60 dosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Cipla Europe NV
De Keyserlei 60C, Bus-1301
2018 Amberes
Bélgica
Responsable de la fabricación
AEROPHARM GmbH
François-Mitterrand-Allee 1
07407 Rudolstadt
Alemania
o
Lek Pharmaceuticals d.d.
Verovškova ulica 57
1526 Ljubljana
Eslovenia
o
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben, Sachsen-Anhalt
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Cipla Europe NV sucursal en España,
C/Guzmán el Bueno, 133 Edif Britannia-28003- Madrid
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Bélgica:Airbufo Forspiro 160 microgram/4,5 microgram/inhalatie, inhalatiepoeder,Voorverdeeld
Dinamarca: AirBuFo Forspiro
Finlandia: AEROCOMP Forspiro160 mikrog/4,5 mikrog/annos, inhalaatiojauhe,
Annosteltu
Francia: AirBuFo Forspiro 160 microgrammes/4,5 microgrammes/dose, poudre pourinhalation en récipient unidose
Irlanda: AirBuFo Forspiro 160 microgram/4.5 microgram/dose inhalation powder,pre-dispensed
Italia: AirBuFo Forspiro
Noruega: AirBuFo Forspiro
Portugal: AirBuFo Forspiro
Suecia: Airbufo Forspiro160 mikrog/4,5 mikrog/dos, inhalationspulver, avdelad dos
Fecha de la última revisión de esteprospecto: Febrero 2023
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
Puede acceder a la información detallada y actualizada sobre cómo administrar este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/info/83517.
- País de registro
- Precio medio en farmacia19.78 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BUDESONIDA/FORMOTEROL CIPLA 160 MICROGRAMOS/4,5 MICROGRAMOS/INHALACION POLVO PARA INHALACION (UNIDOSIS)Forma farmacéutica: INHALACIÓN PULMONAR, 160 microgramos / 4,5 microgramosPrincipio activo: formoterol and budesonideFabricante: Teva Pharma B.V.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 320 microgramos / 9 microgramosPrincipio activo: formoterol and budesonideFabricante: Teva Pharma B.V.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 320 microgramos/9 microgramos/dosisPrincipio activo: formoterol and budesonideFabricante: Cipla Europe N.V.Requiere receta
Médicos online para BUDESONIDA/FORMOTEROL CIPLA 160 MICROGRAMOS/4,5 MICROGRAMOS/INHALACION POLVO PARA INHALACION (UNIDOSIS)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BUDESONIDA/FORMOTEROL CIPLA 160 MICROGRAMOS/4,5 MICROGRAMOS/INHALACION POLVO PARA INHALACION (UNIDOSIS), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















