
BUDESONIDA PULMICTAN 200 microgramos/INHALACION SUSPENSION PARA INHALACION EN ENVASE A PRESION


Cómo usar BUDESONIDA PULMICTAN 200 microgramos/INHALACION SUSPENSION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Budesonida Pulmictan 200 microgramos/inhalación y para qué se utiliza
- Qué necesita saber antes de empezar a usar Budesonida Pulmictan 200 microgramos/inhalación
- Cómo usar Budesonida Pulmictan 200 microgramos/inhalación
- Posibles efectos adversos
- Conservación de Budesonida Pulmictan 200 microgramos/inhalación
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
BUDESONIDA PULMICTAN 200 microgramos/inhalación
suspensión para inhalación en envase a presión
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Budesonida Pulmictan 200 microgramos/inhalación y para qué se utiliza.
- Qué necesita saber antes de empezar a usar Budesonida Pulmictan 200 microgramos/inhalación.
- Cómo usar Budesonida Pulmictan 200 microgramos/inhalación.
- Posibles efectos adversos.
- Conservación de Budesonida Pulmictan 200 microgramos/inhalación.
- Contenido del envase e información adicional.
1. Qué es Budesonida Pulmictan 200 microgramos/inhalación y para qué se utiliza
Budesonida Pulmictan 200 microgramos/inhalación contiene el fármaco budesonida. La budesonida pertenece a un grupo de medicamentos llamados glucocorticoides que se emplean para reducir la inflamación.
El asma está causada por una inflamación de las vías respiratorias. La budesonida reduce y previene esta inflamación.
Budesonida Pulmictan 200 microgramos/inhalación se emplea para el tratamiento del asma. Debe emplearse de forma regular tal y como le indique su médico.
Al inspirar a través del inhalador al mismo tiempo que usted libera una dosis, el medicamento alcanzará los pulmones a través del aire inspirado.
2. Qué necesita saber antes de empezar a usar Budesonida Pulmictan 200 microgramos/inhalación
No use Budesonida Pulmictan 200 microgramos/inhalación
- Si es alérgico a la budesonida o a cualquiera de los componentes de Budesonida Pulmictan 200 microgramos/inhalación.
Informe a su médico para que su medicamento pueda cambiarse por otro.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Budesonida Pulmictan 200 microgramos/inhalación.
Informe a su médico si:
- está tomando o ha tomado recientemente otros medicamentos, incluso los adquiridos sin receta.
- alguna vez ha presentado alguna reacción inusual a Budesonida Pulmictan 200 microgramos/inhalación (budesonida) o a cualquiera de los componentes, o a otros medicamentos.
- usted tiene o ha tenido tuberculosis pulmonar.
- alguna vez ha tenido problemas de hígado.
- tiene una infección bacteriana, vírica o fúngica no tratada en su boca, vías respiratorias o pulmones.
- su médico le ha recetado Budesonida Pulmictan 200 microgramos/inhalación y está todavía bajo tratamiento con comprimidos de corticoides, puede reducirle la dosis de estos comprimidos gradualmente (durante un periodo de semanas o meses) y puede que interrumpa finalmente el tratamiento anterior.
En ese caso, puede que reaparezcan temporalmente algunos síntomas como goteo nasal, urticaria o dolor en los músculos y articulaciones. Si alguno de estos síntomas le preocupa, o presenta algún otro como dolor de cabeza, cansancio, náuseas o vómitos, póngase en contacto con su médico.
Pongase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales
Enjuáguese la boca con agua después de cada inhalación para evitar infección por hongos en la boca.
Budesonida Pulmictan 200 microgramos/inhalación le ha sido recetado para el tratamiento de mantenimiento del asma. Sin embargo, no aliviará un ataque agudo de asma una vez iniciado éste.
Otros medicamentos y Budesonida Pulmictan 200 microgramos/inhalación
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente cualquier otro medicamento, incluso los adquiridos sin receta médica.
Algunos medicamentos pueden aumentar los efectos de Budesonida Pulmictan 200 microgramos/inhalación, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH : ritonavir, cobicistat).
Informe a su médico si usted está usando:
- medicamentos nasales que contienen corticoesteroides
- comprimidos de corticoesteroides
- medicamentos antifúngicos conteniendo ketoconazol e itraconazol
- cimetidina (medicamento para la acidez del estómago)
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Solo se administrará este medicamento durante el embarazo o la lactancia cuando, a criterio médico, el beneficio esperado para la madre sea mayor que cualquier posible riesgo para el feto.
Conducción y uso de máquinas
Budesonida Pulmictan 200 microgramos/inhalación no afecta a su capacidad de conducir ni de utilizar herramientas o máquinas.
Advertencia a los deportistas
Se informa a los deportistas que este medicamento contiene un componente que puede producir un resultado positivo en las pruebas de control de dopaje.
Budesonida Pulmictan 200 microgramos/inhalacióncontieneetanol
Este medicamento contiene 0,33% de etanol (alcohol), esta pequeña cantidad se corresponde con 0,20 mg/dosis.
3. Cómo usar Budesonida Pulmictan 200 microgramos/inhalación
Siga exactamente estas instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico le ha indicado la duración de su tratamiento con Budesonida Pulmictan 200 microgramos/inhalación. No suspenda el tratamiento antes de que su médico se lo diga. No se administre más dosis de las que su médico le ha indicado. Consulte con él cualquier duda que le surja sobre el tratamiento.
Forma de uso y vía de administración
Antes de iniciar el tratamiento deberá conocer el funcionamiento del inhalador. Es importante que usted lea la información incluida en el apartado “Instrucciones para la correcta administración del medicamento” sobre la preparación, utilización y limpieza del inhalador y siga las instrucciones cuidadosamente.
Recuerde enjuagarse siempre la boca con agua después de cada inhalación.
La dosis de Budesonida Pulmictan 200 microgramos/inhalación debe ser individualizada. Su médico le ajustará la dosis y le recetará la mínima que controle sus síntomas del asma. Siga cuidadosamente las instrucciones de su médico.
Uso en adultos
Dosis recomendada habitualmente en adultos: 1 inhalación (200 microgramos) - 8 inhalaciones
(1.600 microgramos) al día, divididas en 2-4 administraciones.
Dosis recomendada habitualmente en ancianos: la misma dosis que los adultos.
Uso en niños y adolescentes
Dosis recomendada habitualmente en niños a partir de 7 años: 1 inhalación (200 microgramos) 4 inhalaciones (800 microgramos) al día, divididas en 2-4 administraciones.
Dosis recomendada habitualmente en niños de 2-7 años: 1 inhalación (200 microgramos) - 2 inhalaciones (400 microgramos) al día, divididas en 2-4 administraciones.
La administración de Budesonida Pulmictan 200 microgramos/inhalación en niños será supervisada por un adulto con el fin de asegurar que la dosis se administra correctamente y de acuerdo a las instrucciones del médico.
Es posible que note una mejoría de los síntomas incluso durante el primer día de tratamiento con Budesonida Pulmictan 200 microgramos/inhalación, aunque pueden requerirse de 1 a 2 semanas antes de alcanzar un efecto completo. Por ello, es importante que no deje de utilizar Budesonida Pulmictan 200 microgramos/inhalación incluso cuando ya se sienta bien.
En adición al preventivo Budesonida Pulmictan 200 microgramos/inhalación, usted puede necesitar también un broncodilatador calmante:
Budesonida NO detendrá un ataque de asma una vez que ya se ha iniciado. Por ello es necesario que siempre tenga a mano un broncodilatador de acción rápida calmante (un agonista beta2) por si presenta síntomas agudos de asma.
Si usted usa un inhalador calmante (un agonista beta2), debería inhalarlo antes de Budesonida Pulmictan 200 microgramos/inhalación (preventivo).
Empeoramiento de los síntomas de asma durante el tratamiento:
Consulte a su médico tan pronto como sea posible si:
- Su sibilancia (jadeo) o dolor en el pecho empeora durante el tratamiento
- Necesita usar el inhalador calmante más a menudo que antes
- Su inhalador calmante no le alivia igual que antes
Su asma puede empeorar y es posible que necesite un tratamiento adicional.
Instrucciones para la correcta administración del medicamento
Preparación del inhalador para su utilización:
Quitar la tapa blanca. En caso de que sea un inhalador nuevo o no se haya utilizado durante varios días, agitar el aerosol y efectuar una pulsación para asegurar el buen funcionamiento del inhalador. En caso de que el inhalador se utilice regularmente pasar a las instrucciones de utilización.
Utilización del inhalador
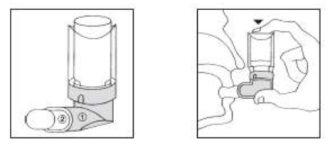
Inhalación con el aerosol solo
- Comprobar que el aerosol se encuentre bien ensamblado al aplicador bucal de plástico (1). Agitar el conjunto y retirar la tapa blanca (2).
- Sostener el frasco en posición invertida entre los dedos pulgar e índice. Introducir la boquilla en la boca apretando los labios alrededor de la misma.
- Realizar una espiración profunda (expulsar el aire por la nariz) y seguidamente efectuar una inspiración a fondo por la boca, presionando al mismo tiempo el frasco entre los dedos y provocando una sola descarga.
- Retirar el aparato de la boca y retener el aire inspirando unos segundos. Espirar lentamente y guardar el frasco colocando la tapa (2) otra vez.
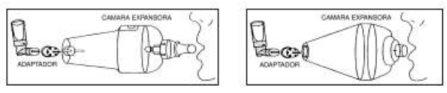
Inhalación con cámara expansora
- Comprobar que el aerosol se encuentre bien ensamblado al aplicador de plástico. Agitar el conjunto y retirar la tapa blanca.
- Si es necesario, acoplar el adaptador a la boquilla del aplicador.
- Ajustar el adaptador, o bien directamente el aplicador, al extremo de la cámara expansora.
- Sujetar la cámara expansora con la boquilla orientada hacia la boca. Presionar el frasco entre los dedos pulgar e índice, provocando la liberación de la dosis dentro de la cámara.
- Espirar profundamente y seguidamente introducir la boquilla de la cámara expansora en la boca, apretando los labios alrededor de la misma.
- Inspirar a fondo por la boca. Retener la respiración unos diez segundos antes de espirar a través de la boquilla de la cámara.
- Inspirar de nuevo profundamente, para asegurar la total inhalación de la dosis administrada. Retener el aire unos segundos y espirar.
- Desacoplar el aerosol de la cámara y del adaptador y guardar el frasco colocando la tapa blanca otra vez.
Limpieza
El pulsador-aplicador bucal debe limpiarse regularmente (al menos una vez a la semana).
- Retirar el pulsador del aerosol y enjuagar con abundante agua.
- Guardar con la tapa colocada, para protegerlo del polvo y la suciedad.
Si usa más Budesonida Pulmictan 200 microgramos/inhalación del que debe
Si usted usa una dosis de Budesonida Pulmictan 200 microgramos/inhalación mayor de la que debe en una sola ocasión, no es probable que se produzcan efectos perjudiciales. Si ha usado demasiado Budesonida Pulmictan 200 microgramos/inhalación durante un periodo largo (meses) es posible que aparezcan efectos adversos. En ese caso, consulte inmediatamente a su médico o a su farmacéutico.
En caso de sobredosis o ingestión accidental, consultar al Servicio de Información Toxicológica. Teléfono 91 562 04 20.
Si olvidó usar Budesonida Pulmictan 200 microgramos/inhalación
Si usted olvida usar alguna de las dosis de Budesonida Pulmictan, no use una dosis doble para compensar las dosis olvidadas. Continúe con el tratamiento habitual tal y como se lo haya prescrito su médico.
Si interrumpe el tratamiento con Budesonida Pulmictan 200 microgramos/inhalación
No interrumpa el tratamiento con Budesonida Pulmictan 200 microgramos/inhalación sin consultar a su médico. Si deja de usar el medicamento bruscamente, su asma puede empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos frecuentes:pueden afectar hasta 1 de cada 10 personas
- Irritación leve de garganta
- Dificultad para tragar
- Tos
- Infección por hongos de la boca y garganta (muguet)
Para prevenir los efectos adversos anteriormente mencionados, se puede enjuagar la boca y la garganta con agua o cepillarse los dientes después de cada dosis. No se trague el agua de los enjuagues, escúpala.
Efectos adversos poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- Ansiedad
- Depresión
- Cataratas
- Visión borrosa
- Espasmos musculares y temblores
Efectos adversos raros:pueden afectar hasta 1 de cada 1.000 personas
- Poco o demasiado cortisol en sangre
- Glándula suprarrenal hipoactiva (glándula cercana a los riñones)
- Erupciones cutáneas, picor, moratones
- Disfonía
- Ansiedad, nerviosismo
- Retraso del crecimiento y cambio de comportamiento en niños
Reacción alérgica aguda rara:
Si poco después de tomar una dosis aparece picor, erupción, enrojecimiento cutáneo, hinchazón de los párpados, los labios, la cara o la garganta, sibilancias, baja persión arterial o colapso, actúe de la siguiente manera:
- Deje de tomar Budesonida Pulmictan 200 microgramos/inhalación
- Busque consejo médico inmediatamente
Falta de aliento inmediatamente después de la dosis:
Raramente, los medicamentos inhalados pueden causar un aumento de las sibilancias y de falta de aliento (broncoespasmo) inmediatamente después de la dosis. Si esto ocurre:
- Deje de tomar Budesonida Pulmictan 200 microgramos/inhalación
- Tome rápidamente un broncodilatador de acción rápida
- Busque consejo médico inmediatamente
Efectos adversos muy raros: pueden afectar hasta 1 de cada 10.000 personas
- Glaucoma
- Disminución de la densidad ósea (debilitamiento de los huesos)
Efectos adversos de frecuencia no conocida(no puede estimarse la frecuencia a partir de los datos disponibles):
- Alteraciones del sueño
- Reacciones agresivas
- Aumento de la actividad motora (dificultad de estarse quieto)
- Irritabilidad
Estos efectos son más probables que aparezcan en niños.
Si previamente estaba bajo tratamiento con comprimidos de corticoides, el paso al tratamiento con corticoides inhalados puede provocar la aparición de algunos síntomas como cansancio, dolor abdominal, debilidad o vómitos. En caso de aparición de estos síntomas, consulte inmediatamente a su médico.
Si cree que puede tener alguno de estos efectos adversos, o si está preocupado por la posibilidad de tenerlos, consulte a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Budesonida Pulmictan 200 microgramos/inhalación
Mantener este medicamento fuera de la vista y el alcance de los niños.
El envase que contiene su medicamento se llena a presión. La válvula no debe sufrir desperfectos, y el envase no debe ser expuesto a temperaturas elevadas o a la luz directa del sol. Asimismo, no se debe perforar, romper o quemar el envase aunque esté vacío.
No refrigerar ni congelar. Conservar por debajo de 30 ºC. Nunca debe exponerse el envase a temperaturas superiores a 50 ºC.
Mantener con la válvula hacia abajo.
Coloque siempre la tapa protectora sobre la boquilla después de utilizar el inhalador.
No utilice Budesonida Pulmictan 200 microgramos/inhalación después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Budesonida Pulmictan 200 microgramos/inhalación
- El principio activo de Budesonida Pulmictan 200 microgramos/inhalación es budesonida. Cada dosis (1 inhalación) contiene 200 microgramos de budesonida.
- Los demás componentes (excipientes) son: ácido oleico, etanol y 1,1,1,2-tetrafluoroetano (HFA 134a).
Aspecto del producto y contenido del envase
Budesonida Pulmictan 200 microgramos/inhalación es una suspensión para ser inhalada a través de un envase a presión.
Cada envase de 5 ml contiene alrededor de 100 dosis.
Cada envase de 10 ml contiene alrededor de 200 dosis.
Existen dos concentraciones de Budesonida Pulmictan en envase a presión: Budesonida Pulmictan 200 microgramos/inhalación y Budesonida Pulmictan Infantil 50 microgramos/inhalación.
Titular de la autorización de comercialización y responsable de la fabricación:
Titular de la autorización de comercialización:
LABORATORIO REIG JOFRÉ, S.A.
Avda. Gran Capità, 10
08970 – Sant Joan Despí
Barcelona, España
Responsable de la fabricación
LABORATORIO REIG JOFRÉ, S.A.
Avda. Gran Capità, 10
08970 – Sant Joan Despí
Barcelona, España
O
GENETIC S.P.A.
Contrada Canfora, Fisciano, Salerno
Italia
Fecha de la última revisión de este prospecto: febrero 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia11.11 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BUDESONIDA PULMICTAN 200 microgramos/INHALACION SUSPENSION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 0,25 mg/mlPrincipio activo: BudesonidaFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/mlPrincipio activo: BudesonidaFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,04000 gPrincipio activo: BudesonidaFabricante: Laboratorio Aldo Union S.L.Requiere receta
Médicos online para BUDESONIDA PULMICTAN 200 microgramos/INHALACION SUSPENSION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BUDESONIDA PULMICTAN 200 microgramos/INHALACION SUSPENSION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















