BOOSTRIX POLIO SUSPENSION INYECTABLE EN JERINGA PRECARGADA

Cómo usar BOOSTRIX POLIO SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Boostrix Polio, Suspensión inyectable en jeringa precargada
Vacuna antidiftérica, antitetánica, antitos ferina (componente acelular) y antipoliomielitis (inactivada) (adsorbida, contenido antigénico reducido)
Lea todo el prospecto detenidamente antes de que usted o su hijo reciba esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted o a su hijo y no debe dársela a otras personas.
- Si usted o su hijo experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Boostrix Polio y para qué se utiliza
- Qué necesita saber antes de que usted o su hijo reciba Boostrix Polio
- Cómo usar Boostrix Polio
- Posibles efectos adversos
- Conservación de Boostrix Polio
- Contenido del envase e información adicional
1. Qué es Boostrix Polio y para qué se utiliza
Boostrix Polio es una vacuna indicada para la vacunación de recuerdo en niños a partir de 3 años, adolescentes y adultos para prevenir cuatro enfermedades: difteria, tétanos (rigidez de mandíbula), tos ferina y poliomielitis (polio). La vacuna actúa ayudando al organismo a producir su propia protección (anticuerpos) frente a estas enfermedades.
- Difteria:La difteria afecta principalmente a las vías respiratorias y algunas veces a la piel. Generalmente, las vías respiratorias se inflaman (se hinchan) causando dificultades respiratorias graves y algunas veces asfixia. La bacteria también libera una toxina (veneno), que puede causar lesiones neurálgicas, problemas cardíacos e incluso la muerte.
- Tétanos (rigidez de mandíbula):La bacteria del tétanos penetra en el organismo a través de cortes, arañazos o heridas en la piel. Las heridas especialmente propensas a la infección son las quemaduras, fracturas, heridas profundas o heridas contaminadas con suciedad, polvo, excrementos de caballo/estiércol o astillas de madera. La bacteria libera una toxina (veneno) que puede causar rigidez muscular, espasmos musculares dolorosos, convulsiones e incluso la muerte. Los espasmos musculares pueden ser tan fuertes que causen fracturas de la espina dorsal.
- Tos ferina:La tos ferina es una afección altamente infecciosa. La enfermedad afecta a las vías respiratorias causando ataques de tos graves que pueden interferir con la respiración normal. La tos se acompaña a menudo de un “aullido” de ahí el nombre común de tos ferina. La tos puede durar 1-2 meses o más tiempo. También puede provocar infecciones de oído, bronquitis que puede durar largo tiempo, neumonía, convulsiones, daño cerebral e incluso la muerte.
- Poliomielitis(polio): La poliomielitis, algunas veces llamada simplemente “polio” es una infección por un virus que puede tener efectos diferentes. A menudo provoca una enfermedad leve pero en algunas personas causa un daño permanente o incluso la muerte. En su forma más grave, la infección de la polio causa parálisis de los músculos (los músculos no se pueden mover), incluyendo aquellos músculos necesarios para respirar y caminar. Los miembros afectados por la enfermedad se pueden deformar de forma dolorosa.
Ninguno de los componentes de la vacuna puede causar difteria, tétanos, tos ferina o poliomielitis.
El uso de Boostrix Polio durante el embarazo ayudará a proteger a su bebé de la tos ferina en los primeros meses de vida, antes de que reciba la inmunización primaria.
2. Qué necesita saber antes de que usted o su hijo reciba Boostrix Polio
No se debe administrar Boostrix Polio:
- si usted o su hijo ha tenido previamente cualquier reacción alérgica a Boostrix Polio o a alguno de los demás componentes de esta vacuna (incluidos en la sección 6), o a la neomicina, la polimixina (antibióticos) o el formaldehído. Los signos de una reacción alérgica pueden incluir, erupción cutánea con picor, dificultad al respirar e inflamación de la cara o lengua
- si usted o su hijo ha tenido previamente una reacción alérgica a cualquier vacuna frente a difteria, tétanos, tos ferina o poliomielitis
- si usted o su hijo ha sufrido problemas en el sistema nervioso (encefalopatía) en los 7 días posteriores a una vacunación anterior con una vacuna frente a tos ferina
- si usted o su hijo ha sufrido una reducción temporal de plaquetas en sangre (la cual incrementa el riesgo de sangrado o de aparición de moratones) o problemas cerebrales o nerviosos tras una vacunación previa con una vacuna frente a difteria y/o tétanos
- si usted o su hijo ha tenido una infección grave con una temperatura alta (superior a 38ºC). Una infección menor, no debe constituir un problema, pero consulte primero a su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de que usted o su hijo reciba Boostrix Polio:
- si usted o su hijo presentó algún problema tras una administración previa de Boostrix Polio u otra vacuna antitos ferina, especialmente:
- Fiebre (superior a 40ºC) en las 48 horas siguientes a la vacunación
- Colapso o estado similar al “shock” en las 48 horas siguientes a la vacunación
- Llanto inconsolable, persistente de más de 3 horas de duración, producido en las 48 horas siguientes a la vacunación
- Convulsiones, con o sin fiebre, producidas en los 3 días siguientes a la vacunación
- si su hijo sufre una enfermedad cerebral no diagnosticada o progresiva o epilepsia no controlada. La vacuna debe administrarse una vez controlada la enfermedad
- si usted o su hijo tiene problemas de hemorragias o le aparecen moratones con facilidad
- si usted o su hijo tiene tendencia a las convulsiones debidas a la fiebre o si existe algún antecedente familiar de esto
- si usted o su hijo tiene problemas del sistema inmune persistentes debido a cualquier causa (incluyendo infección por VIH). Usted o su hijo podrá recibir Boostrix Polio pero la protección frente a infecciones tras la vacunación no será tan buena como la de pacientes con respuestas inmunes adecuadas frente a infecciones.
Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermera si usted o su hijo se ha desmayado en anteriores ocasiones tras la administración de una inyección.
Como con todas las vacunas, Boostrix Polio puede no proporcionar una protección completa en todos los pacientes vacunados.
Uso de Boostrix Polio con otros medicamentos
Informe a su médico o farmacéutico si usted o su hijo está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento o si ha recibido recientemente alguna otra vacuna.
Boostrix Polio puede administrarse al mismo tiempo que algunas vacunas. Emplear un lugar de inyección distinto para cada tipo de vacuna el sitio de inyección será diferente.
Puede que Boostrix Polio no proporcione una respuesta adecuada si usted o su hijo toma medicamentos que reducen la efectividad de su sistema inmune frente a las infecciones.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de recibir esta vacuna.
Se desconoce si Boostrix Polio pasa a la leche materna. Su médico le informará de los posibles riesgos y beneficios de la administración de Boostrix Polio durante la lactancia.
Conducción y uso de máquinas
Es poco probable que Boostrix Polio tenga algún efecto sobre la capacidad de conducir vehículos y utilizar máquinas.
Boostrix Polio contiene neomicina y polimixina
Esta vacuna contiene neomicina y polimixina (antibióticos). Por favor informe a su médico si usted o su hijo ha tenido una reacción alérgica a estos componentes.
Boostrix Polio contiene ácido para-aminobenzoico, fenilalanina, sodio y potasio
Boostrix Polio contiene ácido para-aminobenzoico. Puede provocar reacciones alérgicas (posiblemente retardadas) y, excepcionalmente broncoespasmo.
Esta vacuna contiene 0,0298 microgramos de fenilalanina en cada unidad de dosis. La fenilalanina puede ser perjudicial en caso de padecer fenilcetonuria (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
Esta vacuna contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
Esta vacuna contiene potasio, menos de 1 mmol (39 mg) por dosis; esto es, esencialmente “exento de potasio”.
3. Cómo usar Boostrix Polio
- Boostrix Polio se administrará como una inyección en el músculo.
- La vacuna nunca debe administrarse por vía intravenosa.
- Usted o su hijo recibirá una única inyección de Boostrix Polio.
- Su médico comprobará si usted o su hijo ha recibido previamente vacunas antidiftérica, antitetánica, antitos ferina y/o antipoliomielitis.
- Boostrix Polio puede usarse en caso de sospecha de infección tetánica, aunque además se deben tomar también otras medidas para reducir el riesgo de manifestación de la enfermedad, como por ejemplo curar la herida y/o aplicar toxina antitetánica.
- Su médico le recomendará repetir la vacunación.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Boostrix Polio puede producir efectos adversos, aunque no todas las personas los sufran.
Como con todas las vacunas inyectables, puede padecer reacciones alérgicas graves (reacciones anafilácticas y anafilactoides) en muy raras ocasiones (hasta en un máximo de 1 de cada 10.000 dosis de la vacuna). Éstas se pueden reconocer por:
- Erupciones cutáneas como prurito o ampollas,
- Hinchazón de ojos y cara,
- Dificultad para respirar o tragar,
- Disminución repentina de la presión arterial y pérdida de consciencia.
Estas reacciones pueden ocurrir antes de abandonar la consulta del médico. Sin embargo, si aprecia cualquiera de estos síntomas en usted o su hijo debe contactar inmediatamente con su médico.
Efectos adversos que han ocurrido durante ensayos clínicos en niños de 4 a 8 años
Muy frecuentes(pueden ocurrir con más de 1 de cada 10 dosis de la vacuna): dolor, enrojecimiento e inflamación en el lugar de la inyección, sensación de sueño.
Frecuentes(pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna): fiebre igual o superior a 37,5ºC (incluyendo fiebre de más de 39ºC), hemorragia, picor y endurecimiento en el lugar de la inyección, hinchazón extensa de la extremidad en que se aplicó la vacuna, pérdida de apetito, irritabilidad, dolor de cabeza.
Poco frecuentes(pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): diarrea, náuseas, vómitos, dolor de estómago, inflamación de las glándulas del cuello, axilas o ingle (linfadenopatía), problemas para dormir, apatía, garganta seca, cansancio.
Coadministración con las vacunas sarampión, rubeóla, paperas (SRP) o sarampión, rubeóla, paperas, varicela (SRPV) en niños de 3-6 años de edad
En estudios en los que Boostrix Polio se administró al mismo tiempo que las vacunas SRP o SRPV, se notificaron frecuentemente erupciones cutáneas e infecciones del tracto respiratorio superior (incluyendo secreción nasal y dolor de garganta). La fiebre, irritabilidad, fatiga, pérdida de apetito y los trastornos gastrointestinales (incluyendo diarrea y vómitos) se notificaron con mayor frecuencia (muy frecuentes) que en los estudios en los que Boostrix Polio se administró solo.
Efectos adversos que han ocurrido durante ensayos clínicos en adultos, adolescentes y en niños a partir de 10 años:
Muy frecuentes(pueden ocurrir con más de 1 de cada 10 dosis de la vacuna): dolor, enrojecimiento e inflamación en el lugar de la inyección, cansancio, dolor de cabeza.
Frecuentes(pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna): fiebre igual o superior a 37,5ºC, moratón, picor, endurecimiento, entumecimiento con calor en el lugar de la inyección, dolor de estómago, náuseas, vómitos.
Poco frecuentes(pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): fiebre superior a 39ºC, hinchazón extensa de la extremidad en la que se aplicó la vacuna, escalofríos, dolor, mareos, dolor en las articulaciones, dolor en los músculos, picor, herpes oral, inflamación de las glándulas del cuello, axilas o ingle (linfadenopatía), pérdida de apetito, cosquilleo o entumecimiento de las manos o los pies (parestesia), somnolencia, asma.
Los siguientes efectos adversos ocurrieron durante el uso rutinario de Boostrix Polio y no son específicos para ningún grupo de edad: colapso o pérdida de conocimiento, hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad para tragar o respirar (angioedema), crisis o ataques (con o sin fiebre), habones (urticaria), debilidad inusual (astenia).
Además, los siguientes efectos adversos se notificaron durante ensayos clínicos con Boostrix (vacuna de recuerdo de GlaxoSmithKline frente a difteria, tétanos y tos ferina):
Efectos adversos que han ocurrido en niños de 4 a 8 años
Poco frecuentes(pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): trastornos de la atención, secreción con picor de los ojos y párpados con costra (conjuntivitis), dolor.
Efectos adversos que han ocurrido en adultos, adolescentes y en niños a partir de 10 años
Muy frecuentes(pueden ocurrir con más de 1 de cada 10 dosis de la vacuna): malestar general.
Frecuentes(pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna): induración o absceso en el lugar de la inyección.
Poco frecuentes(pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): infección del tracto respiratorio superior, dolor de garganta y molestias al tragar (faringitis), desmayo (síncope), tos, diarrea, sudoración excesiva (hiperhidrosis), erupción cutánea, rigidez de las articulaciones, rigidez de los músculos y de las articulaciones, síntomas similares a los de la gripe, tales como fiebre, dolor de garganta, moqueo, tos y escalofríos.
Después de la administración de vacunas antitetánicas, en muy raras ocasiones (hasta en un máximo de 1 de cada 10.000 dosis de la vacuna) se han notificado casos de inflamación temporal de los nervios, que causan dolor, debilidad y parálisis en las extremidades y que a menudo llegan al pecho y a la cara (síndrome de Guillain-Barré).
Comunicación de efectos adversos
Si usted o su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Boostrix Polio
Mantener esta vacuna fuera de la vista y del alcance de los niños.
No utilice esta vacuna después de la fecha de caducidad que aparece en el envase y en la etiqueta de la jeringa precargada después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC).
No congelar. La congelación destruye la vacuna.
Conservar en el embalaje original para protegerla de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Boostrix Polio
- Los principios activos son:
Toxoide diftérico1 no menos de 2 Unidades Internacionales (UI) (2,5 Lf)
Toxoide tetánico1 no menos de 20 Unidades Internacionales (UI) (5 Lf)
Antígenos de Bordetella pertussis
Toxoide pertúsico1 8 microgramos
Hemaglutinina filamentosa1 8 microgramos
Pertactina1 2,5 microgramos
Poliovirus inactivados
Tipo 1 (cepa Mahoney)2 40 unidades de antígeno D
Tipo 2 (cepa MEF-1)2 8 unidades de antígeno D
Tipo 3 (cepa Saukett)2 32 unidades de antígeno D
1adsorbidos en hidróxido de aluminio hidratado (Al(OH)3) 0,3 miligramos Al3+
y fosfato de aluminio (AlPO4) 0,2 miligramos Al3+
2 propagado en células Vero
El hidróxido de aluminio y el fosfato de aluminio se incluyen en la vacuna como adyuvantes.
Los adyuvantes son sustancias incluidas en ciertas vacunas para acelerar, mejorar y/o prolongar el efecto protector de la vacuna.
- Los demás componentes son: Medio 199 (conteniendo aminoácidos (incluyendo fenilalanina), sales minerales (incluyendo sodio y potasio), vitaminas (incluyendo ácido para-aminobenzoico) y otras sustancias), cloruro de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Suspensión inyectable en jeringa precargada.
Boostrix Polio es un líquido blanco, ligeramente lechoso que se presenta en una jeringa precargada (0,5 ml).
Boostrix Polio está disponible en jeringa precargada de 1 dosis con o sin agujas separadas; tamaños de envase de 1 y 10.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
GlaxoSmithKline, S.A.
P.T.M - C/ Severo Ochoa, 2
28760 Tres Cantos
Madrid
Tel: 900 202 700
Responsable de la fabricación
GlaxoSmithKline Biologicals S.A.
Rue de l' Institut 89; 1330 Rixensart
Bélgica
Este medicamento está autorizado en los Estados miembros del Espacio Económico Europeo con los siguientes nombres:
Boostrix Polio:Bélgica, Bulgaria, República Checa, Dinamarca, Alemania, Grecia, España, Islandia, Letonia, Lituania, Luxemburgo, Hungría, Países Bajos, Noruega, Austria, Polonia, Portugal, Eslovenia, Finlandia, Suecia
Boostrix Polio Lag:República Eslovaca
Boostrix Tetra:Francia
IPV-Boostrix:Irlanda, Malta
Polio Boostrix:Italia
Boostrix-IPV:Rumania
Fecha de la última revisión de este prospecto: abril 2023
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
-------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Antes de su uso, la vacuna se debe dejar a temperatura ambiente y se debe agitar bien para obtener una suspensión blanca, turbia y homogénea. Antes de la administración, se debe examinar visualmente la vacuna para observar si existe alguna partícula extraña y/o variación del aspecto físico. En caso de apreciarse alguna de estas circunstancias, no administrar la vacuna.
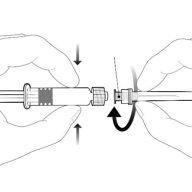
Instrucciones para la jeringa precargada
| Sostenga la jeringa por el cuerpo, no por el émbolo. Desenrosque el tapón de la jeringa girándola en sentido contrario a las agujas del reloj. |
| Para insertar la aguja, conecte la base al adaptador luer-locky gírelo un cuarto de vuelta en el sentido de las agujas del reloj hasta que sienta que se bloquea. No saque el émbolo de la jeringa del cuerpo. Si esto ocurre, no administre la vacuna. |
Eliminación de residuos:
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BOOSTRIX POLIO SUSPENSION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 0,5 mlPrincipio activo: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INYECTABLE, 0,5 mlPrincipio activo: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 1 dosisPrincipio activo: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para BOOSTRIX POLIO SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BOOSTRIX POLIO SUSPENSION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes