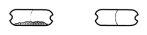BEMRIST BREEZHALER 125 microgramos/62,5 microgramos POLVO PARA INHALACION (CAPSULA DURA)

Cómo usar BEMRIST BREEZHALER 125 microgramos/62,5 microgramos POLVO PARA INHALACION (CAPSULA DURA)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Bemrist Breezhaler 125 microgramos/62,5 microgramos polvo para inhalación (cápsula dura)
Bemrist Breezhaler 125 microgramos/127,5 microgramos polvo para inhalación (cápsula dura)
Bemrist Breezhaler 125 microgramos/260 microgramos polvo para inhalación (cápsula dura)
indacaterol/furoato de mometasona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Bemrist Breezhaler y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bemrist Breezhaler
- Cómo usar Bemrist Breezhaler
- Posibles efectos adversos
- Conservación de Bemrist Breezhaler
- Contenido del envase e información adicional
Instrucciones de uso del inhalador Bemrist Breezhaler
1. Qué es Bemrist Breezhaler y para qué se utiliza
Qué es Bemrist Breezhaler y cómo funciona
Bemrist Breezhaler contiene dos principios activos denominados indacaterol y furoato de mometasona.
Indacaterol pertenece a un grupo de medicamentos llamados broncodilatadores. Relaja los músculos de las vías aéreas pequeñas en los pulmones. Esto ayuda a abrir las vías respiratorias y facilita la entrada y salida de aire de los pulmones. Cuando se usa de forma regular, ayuda a que las pequeñas vías aéreas de los pulmones permanezcan abiertas.
El furoato de mometasona pertenece a un grupo de medicamentos llamados corticosteroides (o esteroides). Los corticosteroides reducen la hinchazón e irritación (inflamación) de las pequeñas vías aéreas en los pulmones y de este modo alivian de forma gradual los problemas respiratorios. Los corticosteroides también ayudan a prevenir los ataques de asma.
Para qué se utiliza Bemrist Breezhaler
Bemrist Breezhaler se utiliza como tratamiento regular del asma en adultos y adolescentes (12 años de edad y mayores).
El asma es una enfermedad pulmonar crónica grave en la que los músculos que rodean las vías respiratorias más pequeñas se estrechan (broncoconstricción) y se inflaman. Los síntomas van y vienen e incluyen dificultad para respirar, sibilancias, opresión en el pecho y tos.
Debe usar Bemrist Breezhaler cada día tal y como le indique su médico y no solo cuando tenga problemas respiratorios u otros síntomas del asma. De este modo asegurará el control de su asma de una manera adecuada. No utilice este medicamento para aliviar un ataque repentino de ahogo o sibilancias.
Consulte a su médico si tiene dudas sobre cómo funciona Bemrist Breezhaler o por qué se le ha prescrito este medicamento.
2. Qué necesita saber antes de empezar a usar Bemrist Breezhaler
Siga todas las instrucciones de su médico de manera cuidadosa.
No use Bemrist Breezhaler
- si es alérgico a indacaterol, furoato de mometasona, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si piensa que puede ser alérgico consulte a su médico.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antesde usar Bemrist Breezhaler si cualquiera de las situaciones siguientes le afecta a usted:
- si tiene problemas de corazón incluyendo latido rápido o irregular,
- si tiene problemas de la glándula tiroides,
- si le han dicho alguna vez que tiene diabetes o niveles altos de azúcar en sangre,
- si padece convulsiones o ataques,
- si tiene un nivel bajo de potasio en sangre,
- si tiene problemas renales graves,
- si tiene tuberculosis (TB) pulmonar o cualquier otra infección desde hace tiempo o que no haya sido tratada.
Durante el tratamiento con Bemrist Breezhaler
Interrumpa el uso de este medicamento y obtenga ayuda médica inmediatamentesi padece cualquiera de las siguientes situaciones:
- opresión en el pecho, tos, sibilancias o dificultad para respirar inmediatamente después de usar Bemrist Breezhaler (signos de que el medicamento inesperadamente estrecha las vías respiratorias, conocido como broncoespasmo paradójico),
- dificultad para respirar o tragar, hinchazón de la lengua, labios o cara, erupción cutánea, picazón y urticaria (signos de una reacción alérgica).
Niños y adolescentes
No administre este medicamento a niños menores de 12 años de edad porque no se ha estudiado en este grupo de edad.
Otros medicamentos y Bemrist Breezhaler
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. En particular, informe a su médico o farmacéutico si está usando:
- medicamentos que disminuyen la cantidad de potasio en la sangre. Estos incluyen diuréticos (que aumentan la producción de orina y pueden usarse para tratar la presión sanguínea elevada, p.ej. hidroclorotiazida), otros broncodilatadores como las metilxantinas usados para trastornos respiratorios (p.ej teofilina) o corticosteroides (p.ej. prednisolona).
- antidepresivos tricíclicos o inhibidores de la monoaminooxidasa (medicamentos utilizados para el tratamiento de la depresión).
- cualquier medicamento que pueda ser similar a Bemrist Breezhaler (que contengan sustancias activas parecidas); puesto que su utilización conjunta puede aumentar el riesgo de efectos adversos.
- medicamentos denominados betabloqueantes que se pueden utilizar para la presión sanguínea elevada u otros problemas del corazón (como es propranolol), o para tratar el glaucoma (p.ej. timolol).
- ketoconazol o itraconazol (medicinas empleadas para tratar las infecciones por hongos).
- ritonavir, nelfinavir o cobicistat (medicamentos empleados para tratar infección por VIH).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Su médico le indicará si puede utilizar Bemrist Breezhaler.
Conducción y uso de máquinas
No es probable que este medicamento afecte a su capacidad para conducir y utilizar máquinas.
Bemrist Breezhaler contiene lactosa
Este medicamento contiene aproximadamente 25 mg de lactosa por cápsula. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo usar Bemrist Breezhaler
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cantidad de Bemrist Breezhaler a inhalar
Hay tres dosis diferentes de Bemrist Breezhaler cápsulas. Su médico decidirá cuál es la mejor para usted.
La dosis habitual es inhalar el contenido de una cápsula cada día. Solo necesita inhalar el medicamento una vez al día. No use más dosis de la indicada por su médico.
Debe usar Bemrist Breezhaler cada día, incluso aunque su asma no le moleste.
Cuándo inhalar Bemrist Breezhaler
Inhale Bemrist Breezhaler en el mismo momento cada día. Esto le ayudará a controlar sus síntomas a lo largo del día y de la noche. Ayudará también a recordar su uso.
Cómo inhalar Bemrist Breezhaler
- Bemrist Breezhaler es para uso por vía inhalatoria.
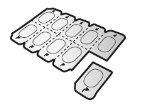
- En este envase, encontrará un inhalador y cápsulas que contienen el medicamento. El inhalador le permite inhalar el medicamento en la cápsula. Utilice las cápsulas únicamente con el inhalador que se proporciona en este envase . Las cápsulas deben mantenerse en el blíster hasta que necesite utilizarlas.
- Despegue la lámina del blíster para abrirlo, no presione la cápsula a través de la lámina.
- Cuando inicie un nuevo envase, use el nuevo inhalador que se proporciona en el envase.
- Deseche el inhalador de cada envase una vez que haya utilizado todas las cápsulas.
- No trague las cápsulas.
- Para más información acerca de cómo usar el inhalador, por favor lea las instrucciones al final de este prospecto.
Si sus síntomas no mejoran
Si su asma no mejora o incluso empeora después de que haya comenzado a usar Bemrist Breezhaler, consulte con su médico.
Si usa más Bemrist Breezhaler del que debe
Si accidentalmente inhala demasiado de este medicamento, contacte con su médico u hospital inmediatamente. Puede ser necesaria atención médica.
Si olvidó usar Bemrist Breezhaler
Si olvidó inhalar una dosis en el horario habitual, inhálela lo más pronto posible en ese mismo día.Luego, el día siguiente inhale la siguiente en el horario habitual. No inhale dos dosis en el mismo día.
Si interrumpe el tratamiento con Bemrist Breezhaler
No interrumpa el uso de Bemrist Breezhaler a menos que su médico se lo indique. Los síntomas de su asma pueden reaparecer si interrumpe su utilización.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves
Interrumpa el uso de Bemrist Breezhaler y obtenga ayuda médica inmediatamente si padece algo de lo siguiente:
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- dificultad para respirar o tragar, hinchazón de la lengua, labios o cara, erupción cutánea, picazón y urticaria (signos de una reacción alérgica).
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas.
- hinchazón de la lengua, labios, cara o garganta (posibles signos de un angioedema)
Otros efectos adversos
A continuación se enumeran otros efectos adversos. Si estos efectos adversos comienzan a ser graves, por favor consulte con su médico, farmacéutico o enfermero.
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- dolor de garganta
- secreción nasal
- dificultad repentina para respirar y sensación de opresión en el pecho con sibilancias o tos
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- alteración de la voz (ronquera)
- congestión nasal
- estornudos, tos
- cefalea
- dolor en los músculos, huesos o articulaciones (signos de dolor musculoesquelético)
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- latido rápido del corazón
- aftas orales (signos de candidiasis)
- altos niveles de azúcar en sangre
- espasmos musculares
- picor
- erupción cutánea
- enturbiamiento en las lentes de sus ojos (signos de catarata)
- visión borrosa
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bemrist Breezhaler
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el blíster después de «CAD»/«EXP». La fecha de caducidad es el último día del mes que se indica.
- No conservar a temperatura superior a 30°C.
- Conservar las cápsulas en el blíster original para protegerlas de la luz y la humedad y no extraerlas hasta justo antes de usar.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Bemrist Breezhaler
- Los principios activos son indacaterol (como acetato) y furoato de mometasona.
Bemrist Breezhaler 125 microgramos/62,5 microgramos
Cada cápsula contiene 173 microgramos de indacaterol como acetato (equivalente a
150 microgramos de indacaterol) y 80 microgramos de furoato de mometasona. La dosis liberada (la dosis que libera la boquilla del inhalador) es equivalente a 125 microgramos de indacaterol y 62,5 microgramos de furoato de mometasona.
Bemrist Breezhaler 125 microgramos/127,5 microgramos
Cada cápsula contiene 173 microgramos de indacaterol como acetato (equivalente a
150 microgramos de indacaterol) y 160 microgramos de furoato de mometasona. La dosis liberada (la dosis que libera la boquilla del inhalador) es equivalente a 125 microgramos de indacaterol y 127,5 microgramos de furoato de mometasona.
Bemrist Breezhaler 125 microgramos/260 microgramos
Cada cápsula contiene 173 microgramos de indacaterol como acetato (equivalente a
150 microgramos de indacaterol) y 320 microgramos de furoato de mometasona. La dosis liberada (la dosis que libera la boquilla del inhalador) es equivalente a 125 microgramos de indacaterol y 260 microgramos de furoato de mometasona.
- El otro componente del polvo para inhalación es (ver el epígrafe “Bemrist Breezhaler contiene lactosa” en la sección 2).
Aspecto del producto y contenido del envase
En este envase, encontrará un inhalador junto con cápsulas en blísteres. Las cápsulas son transparentes y contienen un polvo blanco.
- Las cápsulas de Bemrist Breezhaler 125 microgramos/62,5 microgramos tienen un código de producto “IM150-80” impreso en azul encima de una barra azul en el cuerpo y el logo impreso en azul y rodeado por dos barras azules en la tapa.
- Las cápsulas de Bemrist Breezhaler 125 microgramos/127,5 microgramos tienen un código de producto “IM150-160” impreso en gris en el cuerpo y un logo impreso en gris en la tapa.
- Las cápsulas de Bemrist Breezhaler 125 microgramos/260 microgramos tienen un código de producto “IM150-320” impreso en negro encima de dos barras negras en el cuerpo y un logo impreso en negro y rodeado por dos barras negras en la tapa.
Están disponibles los siguientes tamaños de envase:
Envase unitario conteniendo 10 x 1 o 30 x 1 cápsulas duras, junto con 1 inhalador.
Envases múltiples que contienen 3 cajas, cada una con 30 x 1 cápsulas junto con 3 inhaladores.
Envases múltiples que contienen 15 cajas, cada una con 10 x 1 cápsulas junto con 1 inhalador.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublín 4
Irlanda
Responsable de la fabricación
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Alemania
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
???????? Novartis Bulgaria EOOD ???: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλ?δα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Laboratorios Gebro Pharma, S.A. Tel: +34 93 205 86 86 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κ?προς Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom(Northern Ireland) Novartis Ireland Limited Tel: +44 1276 698370 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
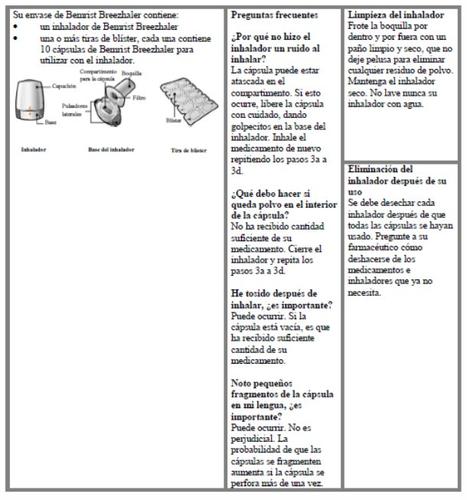
Instrucciones de uso del inhalador Bemrist Breezhaler
Por favor lea las Instrucciones de Uso completas del inhalador Bemrist Breezhaler antes de su utilización.
|
|
|
|
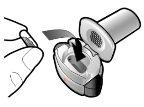
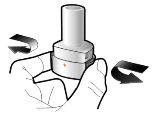
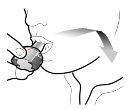
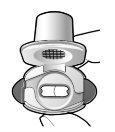
Introducir | Perforar y soltar | Inhalar profundamente | Comprobar que la cápsula esté vacía |
|
|
|
|
|
|
|
|
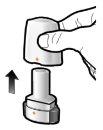
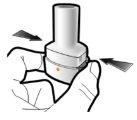
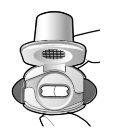
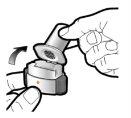
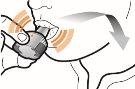
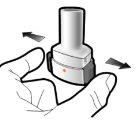
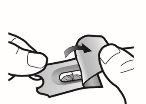
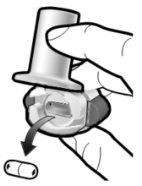
Paso 1a: Retire el capuchón | Paso 2a: Perfore la cápsula una sola vez Sujete el inhalador en posición vertical. Perfore la cápsula presionando firmemente ambos pulsadores al mismo tiempo. | Paso 3a: Espire completamente No sople dentro del inhalador. | Comprobar que la cápsula está vacía Abra el inhalador para comprobar si queda polvo en la cápsula. Si queda polvo en la cápsula:
|
| Deberá oír un ruido cuando se perfore la cápsula. Perfore la cápsula sólo una vez. |
|
Queda polvoVacía |
Paso 1b: Abra el inhalador |
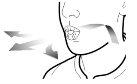
Paso 2b: Suelte completamente los pulsadores | Paso 3b: Inhale el medicamento profundamente Sujete el inhalador como se muestra en la figura. Introduzca la boquilla en su boca y cierre los labios firmemente en torno a ella. No presione los pulsadores. | |
| Inspire de forma rápida y tan profundamente como pueda. Durante la inhalación oirá un zumbido. Puede notar el gusto del medicamento cuando inhale. |
| |
Paso 1c: Extraiga la cápsula Separe uno de los blísteres de la tira del blíster. Abra el blíster y extraiga una cápsula. No presione la cápsula a través de la lámina. No trague la cápsula. |
Paso 3c: Contenga la respiración Contenga la respiración durante 5 segundos. Paso 3d: Enjuague la boca Enjuague su boca con agua después de cada dosis y escúpala. | Extraiga la cápsula vacía Deseche la cápsula vacía en la basura de su casa. Cierre el inhalador y coloque de nuevo el capuchón. | |
Paso 1d: Introduzca la cápsula No coloque nunca la cápsula directamente en la boquilla. | Información importante
| ||
Paso 1e: Cierre el inhalador |
- País de registro
- Precio medio en farmacia41.28 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BEMRIST BREEZHALER 125 microgramos/62,5 microgramos POLVO PARA INHALACION (CAPSULA DURA)Forma farmacéutica: INHALACIÓN PULMONAR, 125 microgramos/127.5 microgramosPrincipio activo: indacaterol and mometasoneFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 125/260 µgPrincipio activo: indacaterol and mometasoneFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 125 microgramos/62.5 microgramosPrincipio activo: indacaterol and mometasoneFabricante: Novartis Europharm LimitedRequiere receta
Médicos online para BEMRIST BREEZHALER 125 microgramos/62,5 microgramos POLVO PARA INHALACION (CAPSULA DURA)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BEMRIST BREEZHALER 125 microgramos/62,5 microgramos POLVO PARA INHALACION (CAPSULA DURA), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes