
BEMFOLA 225UI/0,375ml solucion inyectable en pluma precargada


Cómo usar BEMFOLA 225UI/0,375ml solucion inyectable en pluma precargada
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Bemfola 75 UI/0,125 ml solución inyectable en pluma precargada
Bemfola 150 UI/0,25 ml solución inyectable en pluma precargada
Bemfola 225 UI/0,375 ml solución inyectable en pluma precargada
Bemfola 300 UI/0,50 ml solución inyectable en pluma precargada
Bemfola 450 UI/0,75 ml solución inyectable en pluma precargada
Folitropina alfa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Bemfola y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bemfola
- Cómo usar Bemfola
- Posibles efectos adversos
- Conservación de Bemfola
- Contenido del envase e información adicional
1. Qué es Bemfola y para qué se utiliza
Qué es Bemfola
Este medicamento contiene el principio activo folitropina alfa, que es casi idéntico a una hormona natural producida por su organismo que se denomina «hormona foliculoestimulante» (FSH). La FSH es una gonadotropina, un tipo de hormona que desempeña un papel importante en la reproducción y la fertilidad humana. En mujeres, la FSH es necesaria para el crecimiento y desarrollo de los sacos (folículos) de los ovarios que contienen los óvulos. En varones, la FSH es necesaria para la producción de esperma.
Para qué se utiliza Bemfola
En mujeres adultas,Bemfola se utiliza:
- para ayudar a liberar un óvulo del ovario (ovulación) en mujeres que no pueden ovular y que no han respondido al tratamiento con una sustancia llamada «citrato de clomifeno»
- junto con otra sustancia llamada «lutropina alfa» («hormona luteinizante» o LH), para ayudar a liberar un óvulo del ovario (ovulación) en mujeres cuyo organismo produce cantidades muy pequeñas de gonadotropinas (FSH y LH).
- para ayudar a desarrollar varios folículos (que contiene cada uno un óvulo) en mujeres que se someten a técnicas de reproducción asistida (técnicas que pueden ayudarla a quedarse embarazada), como la «fertilización in vitro», la «transferencia intratubárica de gametos» o la
«transferencia intratubárica de cigotos».
En varones adultos,Bemfola se utiliza:
- junto con otro medicamento llamado «Gonadotropina Coriónica humana» (hCG), para ayudar a producir esperma en varones que son estériles debido a una concentración baja de ciertas hormonas.
2. Qué necesita saber antes de empezar a usar Bemfola
Antes de iniciar el tratamiento se debe valorar su fertilidad y la de su pareja por parte de un médico experimentado en el tratamiento de los trastornos de la fertilidad.
No use Bemfola
- si es alérgico a la hormona foliculoestimulante o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene un tumor en el hipotálamo o en la hipófisis (ambos son partes del cerebro).
- si usted es una mujer:
- con ovarios grandes o bolsas de líquido en el interior de los ovarios (quistes ováricos) de origen desconocido.
- con hemorragia vaginal inexplicada.
- con cáncer de ovario, de útero o de mama.
- con una afección que normalmente hace que el embarazo sea imposible, como la insuficiencia ovárica ( menopausia precoz) o una malformación de los órganos reproductivos.
- si usted es un varón:
- con testículos dañados que no pueden curarse.
No utilice Bemfola si sufre alguna de estas afecciones. Si no está seguro, pregunte a su médico antes de usar este medicamento.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de utilizar Bemfola.
Porfiria
Informe a su médico antes de iniciar el tratamiento, si usted o cualquier miembro de su familia padecen porfiria (una incapacidad para degradar las porfirinas que puede transmitirse de padres a hijos).
Informe inmediatamente a su médico si:
- su piel se vuelve frágil y le salen ampollas con facilidad, especialmente en las zonas expuestas al sol con frecuencia, y/o
- si tiene dolor de estómago, de brazos o piernas.
En estos casos, su médico puede recomendarle que interrumpa el tratamiento.
Síndrome de hiperestimulación ovárica (SHO)
Si es usted una mujer, este medicamento aumenta el riesgo de que presente un SHO. Esto ocurre cuando sus folículos se desarrollan demasiado y se convierten en quistes de gran tamaño. Si tiene dolor en la región pélvica, aumenta de peso rápidamente, tiene náuseas o vómitos o dificultad para respirar, consulte inmediatamente con su médico, quien puede interrumpir el tratamiento (ver la sección 4).
En caso de que no ovule y se respeten la dosis y el esquema posológico recomendados, ese síndrome es menos probable que ocurra. El tratamiento con Bemfola rara vez causa un síndrome de hiperestimulación ovárica grave, a menos que se administre el medicamento que se usa para la maduración folicular final (que contiene Gonadotropina Coriónica humana, hCG). En caso de desarrollar SHO, su médico puede no recetarle hCG en este ciclo de tratamiento y aconsejarle que se abstenga de realizar el coito o que utilice métodos anticonceptivos de barrera durante al menos 4 días.
Embarazo múltiple
Si usa Bemfola, tiene un riesgo más alto de quedarse embarazada de más de un niño a la vez («embarazo múltiple», generalmente gemelos), que si se queda embarazada por concepción natural. El embarazo múltiple puede causar complicaciones médicas para usted y sus bebés. Usted puede reducir el riesgo de embarazo múltiple usando la dosis correcta de Bemfola a las horas correctas. Si se somete
a técnicas de reproducción asistida, el riesgo de embarazo múltiple está relacionado con su edad y con la calidad y el número de óvulos fertilizados o embriones que se coloquen en su interior.
Aborto
Si se somete a técnicas de reproducción asistida o a estimulación de sus ovarios para producir óvulos, es más probable que tenga un aborto que en el promedio de las mujeres.
Problemas de coagulación de la sangre (episodios tromboembólicos)
Si usted o algún miembro de su familia ha sufrido, en el pasado o recientemente, coágulos de sangre en la pierna o en el pulmón, infarto de miocardio o ictus, usted podría tener un riesgo más alto de presentar estos problemas o de que empeorasen con el tratamiento con Bemfola.
Varones con niveles altos de FSH en la sangre
Si usted es varón, unos niveles demasiado altos de FSH en la sangre pueden ser un signo de lesión de los testículos. Por lo general, Bemfola no suele ser eficaz en estos casos.
Si su médico decide intentar el tratamiento con Bemfola para controlar el tratamiento, su médico puede pedirle que se haga un análisis de semen, de 4 a 6 meses después de iniciar el tratamiento.
Niños y adolescentes
Bemfola no está indicado en niños y adolescentes menores de 18 años.
Uso de Bemfola y otros medicamentos
Informe a su médico si está tomando, ha tomado recientemente o podría tener que tomar otros medicamentos.
- Si usa Bemfola con otros medicamentos que ayudan a la ovulación (por ejemplo, hCG o citrato de clomifeno), la respuesta de sus folículos puede verse aumentada.
- Si usa Bemfola al mismo tiempo que un agonista o un antagonista de la «hormona liberadora de gonadotropinas» (GnRH) (estos medicamentos disminuyen las concentraciones de las hormonas sexuales y detienen la ovulación), puede necesitar una dosis más alta de Bemfola para producir folículos.
Embarazo y lactancia
No use Bemfola si está embarazada o durante el periodo de lactancia.
Conducción y uso de máquinas
No se espera que este medicamento afecte a su capacidad para conducir y utilizar máquinas.
Bemfola contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis, por lo que se considera esencialmente «exento de sodio».
3. Cómo usar Bemfola
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Uso de este medicamento
- Bemfola está diseñado para que se administre mediante inyección justo debajo de la piel (por vía subcutánea). Utilice cada pluma precargada una sóla vez y después deséchela de una forma segura. No administre la solución si contiene partículas o no es transparente.
- La primera inyección de Bemfola se debe administrar bajo la supervisión de su médico.
- Su médico o enfermero le enseñarán cómo usar la pluma precargada de Bemfola para que pueda inyectarse el medicamento usted mismo.
- Si usted se autoadministra Bemfola lea y siga atentamente las «Instrucciones de uso». Estas instrucciones se pueden consultar al final del prospecto.
Qué cantidad se debe usar
Su médico decidirá qué cantidad de medicamento se administrará y con qué frecuencia. Las dosis que se describen a continuación están expresadas en Unidades Internacionales (UI) y mililitros (ml).
Mujeres
Si no está ovulando y tiene menstruaciones irregulares o no tiene menstruación
- Bemfola se administra generalmente todos los días.
- Si usted tiene una menstruación irregular, empiece usando Bemfola los 7 primeros días del ciclo menstrual. Si no tiene menstruación, puede empezar a usar el medicamento cualquier día que le resulte cómodo.
- La dosis inicial habitual de Bemfola es de 75 a 150 UI (0,12 a 0,25 ml) cada día.
- Su dosis de Bemfola puede aumentarse cada 7 ó 14 días en 37,5 a 75 UI, hasta que se obtenga la respuesta.
- La dosis máxima diaria de Bemfola no suele ser mayor de 225 UI (0,375 ml).
- Cuando se obtenga la respuesta deseada, se le administrará una inyección única de
250 microgramos de «hCG recombinante» (hCG-r, una hCG fabricada en un laboratorio mediante una técnica especial de ADN), ó 5.000 a 10.000 UI de hCG, 24 a 48 horas después de la última inyección de Bemfola. El mejor momento para mantener relaciones sexuales es el mismo día de la inyección de hCG y al día siguiente.
Si su médico no observa la respuesta deseada después de 4 semanas de tratamiento, ese ciclo de tratamiento con Bemfola se debe interrumpir. Para el siguiente ciclo, su médico le administrará una dosis inicial más alta de Bemfola que la anterior.
Si se obtiene una respuesta excesiva, se interrumpirá su tratamiento y no le administrarán hCG (ver la sección 2, “Síndrome de hiperestimulación ovárica (SHO)”). Para el siguiente ciclo, su médico le administrará una dosis de Bemfola más baja que la del ciclo previo.
Si se le ha diagnosticado un déficit de hormonas FSH y LH
- La dosis inicial habitual de Bemfola es de 75 a 150 UI (0,12 a 0,25 ml), junto con 75 UI (0,12 ml) de lutropina alfa.
- Usará estos dos medicamentos cada día, hasta un período de cinco semanas.
- La dosis de Bemfola puede aumentarse cada 7 ó 14 días, en 37,5 a 75 UI, hasta que se obtenga la respuesta deseada.
- Cuando se obtenga la respuesta deseada, se le administrará una inyección única de
250 microgramos de «hCG recombinante» (hCG-r, una hCG fabricada en un laboratorio mediante una técnica especial de ADN), ó 5.000 a 10.000 UI de hCG, 24 a 48 horas después de la última inyección de Bemfola y lutropina alfa. El mejor momento para mantener relaciones sexuales es el mismo día de la inyección de hCG y al día siguiente. También, puede realizarse una inseminación intrauterina u otra técnica de reproducción asistida según el criterio de su médico.
Si su médico no observa la respuesta deseada después de cinco semanas, ese ciclo de tratamiento con Bemfola se debe interrumpir. Para el siguiente ciclo, su médico le administrará una dosis inicial más alta de Bemfola que la del ciclo cancelado.
Si se obtiene una respuesta excesiva, se interrumpirá su tratamiento y no le administrarán hCG (ver la sección 2, “Síndrome de hiperestimulación ovárica (SHO)”). Para el siguiente ciclo, su médico le administrará una dosis de Bemfola más baja que la del ciclo previo.
Si tiene que desarrollar varios óvulos para su extracción previamente a cualquier técnica de reproducción asistida
- La dosis inicial habitual de Bemfola es de 150 a 225 UI (0,25 a 0,37 ml) cada día, desde el 2º o 3er día de su ciclo de tratamiento.
- La dosis de Bemfola puede aumentarse, dependiendo de su respuesta. La dosis diaria máxima es de 450 UI (0,75 ml).
- El tratamiento continúa hasta que los óvulos se hayan desarrollado hasta el punto deseado. Esto requiere normalmente unos 10 días, pero puede variar entre 5 y 20 días. Su médico lo comprobará mediante análisis de sangre y/o ecografías.
- Cuando los óvulos estén listos, se le administrará una inyección única de 250 microgramos de«hCG recombinante» (hCG-r, una hCG fabricada en un laboratorio mediante una técnica especial de ADN recombinante), ó 5.000 UI a 10.000 UI de hCG, 24 a 48 horas después de la última inyección de Bemfola. Esto hace que sus óvulos estén listos para su extracción.
En otros casos, su médico puede interrumpir primero la ovulación, mediante el uso de un agonista o un antagonista de la hormona liberadora de gonadotropinas (GnRH). En tales casos, la administración de Bemfola se inicia aproximadamente 2 semanas después de iniciar el tratamiento con el agonista, continuando ambos tratamientos hasta lograr un desarrollo folicular adecuado. Por ejemplo, después de dos semanas de tratamiento con el agonista de la GnRH, se administran de 150 a 225 UI de Bemfola durante 7 días. A continuación, la dosis se ajusta según la respuesta de los ovarios
Varones
- La dosis habitual de Bemfola es de 150 UI (0,25 ml) junto con hCG.
- Usted usará estos dos medicamentos tres veces por semana, durante al menos 4 meses.
- Si no ha respondido al tratamiento después de 4 meses, su médico puede sugerirle que siga usando estos dos medicamentos al menos durante 18 meses.
Si usa más Bemfola del que debiera
Se desconocen los efectos de usar una cantidad excesiva de Bemfola. Sin embargo, puede esperarse que se produzca un síndrome de hiperestimulación ovárica, que se describe en la sección 4. Sin embargo, este síndrome sólo ocurrirá si se administra también hCG (ver la sección 2, “Síndrome de hiperestimulación ovárica (SHO)”).
Si olvidó usar Bemfola
Si olvidó usar Bemfola, no use una dosis doble para compensar las dosis olvidadas. Consulte a su médico tan pronto como se dé cuenta de que se ha olvidado de administrar una dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves en mujeres
- El dolor pélvico, acompañado de náuseas o vómitos, pueden ser síntomas del síndrome de hiperestimulación ovárica (SHO). Esto puede indicar que los ovarios han reaccionado de forma excesiva al tratamiento y se han desarrollado quistes ováricos de gran tamaño (ver también la sección 2 “Síndrome de hiperestimulación ovárica (SHO)”). Este efecto adverso es frecuente (puede afectar hasta 1 de cada 10 personas).
- El síndrome de hiperestimulación ovárica puede agravarse con ovarios claramente aumentados de tamaño, disminución de la producción de orina, aumento de peso, dificultad para respirar y/o posible acumulación de líquido en el abdomen o en el pecho. Este efecto adverso es poco frecuente (puede afectar hasta 1 de cada 100 personas).
- En casos raros, también se pueden producir complicaciones del síndrome de hiperestimulación ovárica como torsión ovárica o coagulación de la sangre (puede afectar hasta 1 de cada 1.000 personas).
- En casos muy raros se pueden producir complicaciones graves de la coagulación de la sangre (episodios tromboembólicos) a veces independientes del síndrome de hiperestimulación ovárica (puede afectar hasta 1 de cada 10.000 personas). Esto podría causar dolor en el pecho, sensación de falta de aire, ictus o infarto de miocardio (ver también la sección 2 «Problemas de coagulación de la sangre (episodios tromboembólicos)»).
Efectos adversos graves en varones y en mujeres
- Las reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar, a veces pueden ser graves. Este efecto adverso es muy raro (pueden afectar hasta a 1 de cada 10.000 personas).
Si observa alguno de los efectos adversos antes mencionados, debe consultar inmediatamente a su médico, quien podría pedirle que interrumpa el tratamiento con Bemfola.
Otros efectos adversos en mujeres
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Bolsas de líquido en el interior de los ovarios (quistes ováricos).
- Dolor de cabeza
- Reacciones locales en el lugar de la inyección, como dolor, enrojecimiento, hematoma, hinchazón y/o irritación.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Dolor abdominal
- Náuseas, vómitos, diarrea, retortijones y flatulencias
Muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- Pueden producirse reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar. En ocasiones, estas reacciones pueden ser graves.
- El asma puede empeorar
Otros efectos adversos en varones
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Reacciones locales en el lugar de la inyección, como dolor, enrojecimiento, hematoma, hinchazón y/o irritación.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Hinchazón de las venas por encima y por detrás de los testículos (varicocele).
- Desarrollo de mamas, acné o aumento de peso.
Muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- Pueden producirse reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar. En ocasiones, estas reacciones pueden ser graves.
- El asma puede empeorar
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bemfola
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta o la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar. Conservar en el embalaje original para protegerlo de la luz.
Durante su periodo de validez, el medicamento no abierto puede conservarse a temperatura igual o inferior a 25 ºC durante un máximo de 3 meses sin refrigerar y se debe desechar si no se ha utilizado en el plazo de 3 meses.
No utilice este medicamento si observa cualquier indicio visible de deterioro, si el líquido contiene partículas o si no es transparente.
Una vez abierto, el medicamento se inyectará inmediatamente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Bemfola
- El principio activo es folitropina alfa.
- Bemfola 75 UI/0,125 ml: cada cartucho contiene 75 UI (equivalente a 5,5 microgramos) de folitropina alfa en 0,125 ml de solución.
- Bemfola 150 UI/0,25 ml: cada cartucho contiene 150 UI (equivalente a 11 microgramos) de folitropina alfa en 0,25 ml de solución.
- Bemfola 225 UI/0,375 ml: cada cartucho contiene 225 UI (equivalente a 16,5 microgramos) de folitropina alfa en 0,375 ml de solución.
- Bemfola 300 UI/0,50 ml: cada cartucho contiene 300 UI (equivalente a 22 microgramos) de folitropina alfa en 0,50 ml de solución.
- Bemfola 450 UI/0,75 ml: cada cartucho contiene 450 UI (equivalente a 33 microgramos) de folitropina alfa en 0,75 ml de solución.
- Cada ml de la solución contiene 600 UI (equivalente a 44 microgramos) de folitropina alfa.
- Los demás componentes son poloxámero 188, sacarosa, metionina, fosfato de disodio dihidrato, dihidrogenofosfato de sodio dihidrato, ácido fosfórico y agua para preparaciones inyectables.
Aspecto de Bemfola y contenido del envase
- Bemfola se presenta como un líquido inyectable, transparente e incoloro, en una pluma precargada.
- Bemfola se suministra en envases de 1, 5 o 10 plumas precargadas, 1, 5 o 10 agujas desechables y 1, 5 o 10 gasas empapadas en alcohol. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungría
Fecha de la última revisión de este prospecto
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Bemfola 75 UI/0,125 ml pluma precargada
Bemfola 150 UI/0,25 ml pluma precargada
Bemfola 225 UI/0,375 ml pluma precargada
Bemfola 300 UI/0,50 ml pluma precargada
Bemfola 450 UI/0,75 ml pluma precargada
Instrucciones de Uso
CONTENIDO
- Cómo usar la pluma precargada de Bemfola
- Antes de comenzar a usar la pluma precargada
- Preparación de la pluma precargada para la inyección
- Ajuste de la dosis prescrita por su médico
- Inyección de la dosis
- Después de la inyección
Advertencia: Lea y siga estas Instrucciones de uso de la pluma precargada de Bemfola. No siga instrucciones de fuentes distintas a las proporcionadas en estas Instrucciones de uso o por un profesional sanitario, ya que puede comprometer el uso adecuado de la pluma precargada y sutratamiento. |
- Cómo usar la pluma precargada de Bemfola
- Antes de empezar a utilizar sus plumas precargadas, lea atentamente las Instrucciones de uso completas y el prospecto.
- Cada pluma precargada es sólo para su uso individual; no deje que nadie más la utilice.
- Los números que figuran en el indicador de dosis de las plumas precargadas se miden en Unidades Internacionales o UI. Su médico le habrá indicado cuántas UI debe inyectarse cada día.
- Su médico/farmacéutico le dirá cuántas plumas precargadas de Bemfola de un solo uso necesita usar para su ciclo de tratamiento completo.
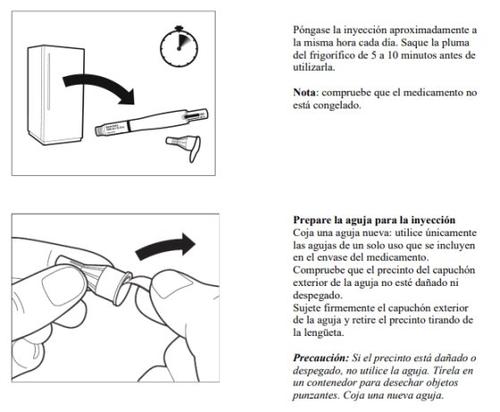
- Póngase la inyección aproximadamente a la misma hora cada día.
- Antes de empezar a utilizar la pluma precargada
- Saque la pluma de la nevera
- Saque la pluma de la nevera de 5 a 10 minutos antes de utilizarla.
- Si el medicamento está congelado, no lo utilice.
- Lávese las manos
- Lávese las manos con agua tibia y jabón, a continuación, séquelas.
- Es importante que las manos y los objetos que utilice para preparar la pluma estén lo más limpios posible.
- Busque un lugar limpio
- Un lugar adecuado es una mesa o superficie limpia.
- Preparación de la pluma precargada para la inyección
Las diferentes partes de su pluma precargada
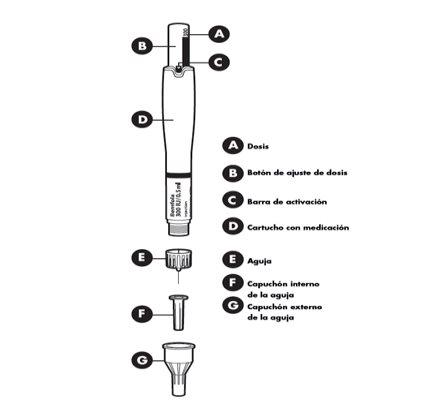
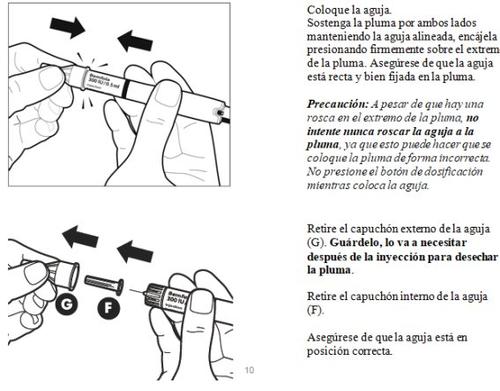
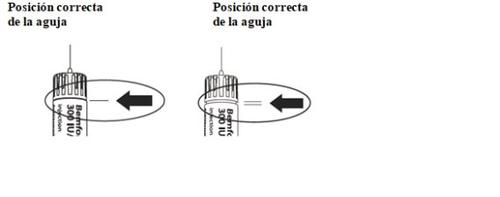
- Ajuste de la dosis prescrita por su médico
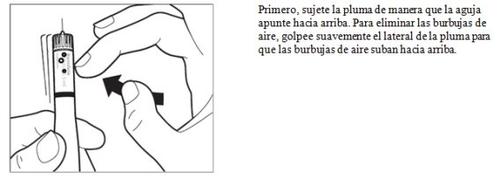
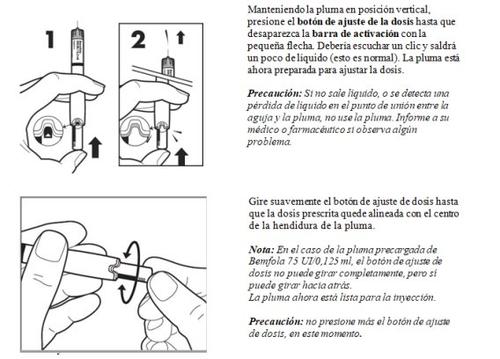
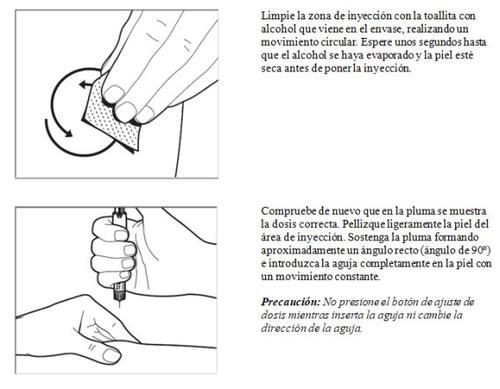
- Inyección de la dosis
Ahora está listo para aplicarse la inyección inmediatamente: su médico o enfermero le habrán indicado dónde debe ponerse la inyección (p. ej., en el vientre, en la parte anterior del muslo). Para minimizar la irritación de la piel, seleccione un lugar de inyección diferente cada día.
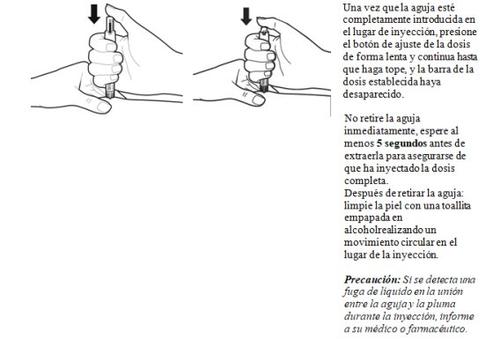
- Después de la inyección
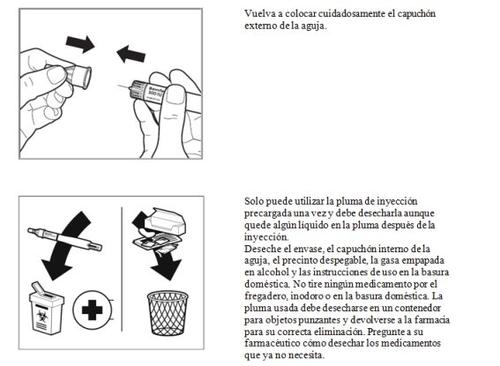
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BEMFOLA 225UI/0,375ml solucion inyectable en pluma precargadaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150UI/0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para BEMFOLA 225UI/0,375ml solucion inyectable en pluma precargada
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BEMFOLA 225UI/0,375ml solucion inyectable en pluma precargada, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






