
BECLO-ASMA 50 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION

Cómo usar BECLO-ASMA 50 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Beclo-Asma 50 microgramos/pulsación y para qué se utiliza
- Qué necesita saber antes de empezar a usar Beclo-Asma 50 microgramos/pulsación
- Cómo usar Beclo-Asma 50 microgramos/pulsación
- Posibles efectos adversos
- Conservación de Beclo-Asma 50 microgramos/pulsación
- Contenido del envase e información adicional
Introducción
Prospecto: información para el paciente
Beclo-Asma 50 microgramos/pulsación solución para inhalación en envase a presión
dipropionato de beclometasona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Beclo-Asma 50 microgramos/pulsación y para qué se utiliza
- Qué necesita saber antes de empezar a usar Beclo-Asma 50 microgramos/pulsación
- Cómo usar Beclo-Asma 50 microgramos/pulsación
- Posibles efectos adversos
- Conservación de Beclo-Asma 50 microgramos/pulsación
- Contenido del envase e información adicional
1. Qué es Beclo-Asma 50 microgramos/pulsación y para qué se utiliza
Beclo-Asma 50 microgramos/pulsación contiene como principio activo dipropionato de beclometasona, que pertenece al grupo de medicamentos llamados corticosteroides. Los corticosteroides se utilizan para el tratamiento del asma ya que tienen entre otras, una acción antiinflamatoria. Reducen la hinchazón e irritación en las paredes de los pequeños conductos por los que circula el aire en los pulmones y facilitan así la respiración.
Beclo-Asma se utiliza para prevenir los síntomas del asma en personas que necesitan tratamiento regular.
Los corticosteroides también ayudan a prevenir los ataques de asma.
No esta indicado para el alivio de broncoespasmo agudo.
2. Qué necesita saber antes de empezar a usar Beclo-Asma 50 microgramos/pulsación
No use Beclo-Asma 50 microgramos/pulsación:
- Si es alérgico al dipropionato de beclometasona o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Para tratar un ataque repentino de dificultad respiratoria.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Beclo-Asma 50 microgramos/pulsación:
- Si ha tenido aftas en la boca
- Si está tomando o ha tomado recientemente algún tipo de comprimido o de inyectables de tipo esteroideo
- Si está siendo o ha sido tratado de tuberculosis.
- Si tiene antecedentes de diabetes mellitus (ya que este medicamento puede aumentar la concentración de glucosa en la sangre),
- Si ha usado dosis elevadas de este medicamento durante un período prolongado de tiempo y experimenta los siguientes síntomas:
- Aumento de peso y redondeo del rostro (cara de luna) (síndrome de Cushing),
- Síntomas imprecisos, como dolor abdominal, náuseas, diarrea, dolor de cabeza o somnolencia (supresión suprarrenal, crisis suprarrenal aguda). Estos síntomas son más probables durante una infección, como las infecciones virales o malestar de estómago,
- Pérdida de masa ósea,
- Problemas oculares (cataratas y glaucoma),
- Retraso del crecimiento (esto sucede principalmente en niños y adolescentes).
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Los pacientes que hayan sido previamente tratados con beclometasona deben saber que este medicamento no contiene propelentes clorofluorocarbonados (CFCs). El principio activo es el mismo. Las únicas diferencias que se pueden notar son el sabor y sensación de notar el spray en la boca, además del sonido del inhalador durante la utilización.
Debe utilizarse exactamente como le indique el médico. Su médico podría cambiarle la pauta posológica.
Uso en deportistas
Se informa a los deportistas que este medicamento contiene beclometasona dipropionato que puede establecer un resultado analítico de control de dopaje como positivo.
Otros medicamentos y Beclo-Asma 50
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tomar cualquier otro medicamento.
Algunos medicamentos pueden aumentar los efectos de Beclo-Asma 50 microgramos/pulsación, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Comunique a su médico si está tomando disulfiram o metronidazol, ya que existe un potencial riesgo de interacción en personas particularmente sensibles.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento
Embarazo: no existe evidencia suficiente sobre la utilización del producto durante el período de gestación.
El uso de beclometasona dipropionato durante el embarazo requiere sopesar los posibles beneficios del fármaco, frente a los riesgos posibles.
Lactancia: es probable que la beclometasona se excrete en la leche materna. Sin embargo, considerando las dosis relativamente bajas utilizadas vía inhalatoria, es probable que los niveles sean bajos. En madres en período de lactancia, deberán sopesarse los beneficios terapéuticos del fármaco frente a los riesgos potenciales para la madre y el bebé.
Conducción y uso de máquinas
Beclo-Asma 50 microgramos/pulsación no tiene efectos conocidos sobre la conducción y el uso de máquinas.
Beclo-Asma 50 microgramos/pulsación contiene etanol
Este medicamento contiene unos 4,7 mg de alcohol (etanol) en cada inhalación. La cantidad por inhalación de este medicamento es equivalente a menos de 1 ml de cerveza o 1 ml de vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible.
.
3. Cómo usar Beclo-Asma 50 microgramos/pulsación
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de dudas, consulte de nuevo a su médico o farmacéutico.
Recuerde usar su medicamento.
Beclo-Asma sólo debe utilizarse por vía inhalatoria.
Su médico le indicará la duración de su tratamiento con Beclo-Asma. No suspenda el tratamiento antes, aun cuando se encuentre mejor, a menos que su médico se lo indique o note que su respiración empeora cuando toma el medicamento. Las dosis habituales recomendadas son las siguientes:
Adultos y niños de más de 12 años
La dosis inicial para asma leve o moderado es de una inhalación(50 microgramos) dos veces al día. Esta dosis puede incrementarse a dos inhalaciones (100 microgramos) dos veces al día.
Para asma más grave la dosis usual es hasta de cuatro inhalaciones (200 microgramos) dos veces al día.
La dosis máxima recomendada es de 800 microgramos al día.
Niños de 6 a 11 años
La dosis debe establecerla el médico y dependiendo de la respuesta individual de cada paciente. En general se aconseja la mitad de las dosis recomendadas para adultos (máximo 400 microgramos al día en dos dosis divididas). Los niños deberían recibir una dosis inicial de Beclo-Asma apropiada a la gravedad de su enfermedad. La dosis puede luego ajustarse hasta que se alcance un control adecuado o reducirse a la mínima dosis eficaz de acuerdo a la respuesta individual.
Si empieza a utilizar Beclo-Asma, en lugar de, o al mismo tiempo que, esteroides por vía oral, debe llevar una tarjeta de aviso de que toma esteroides hasta que su médico le indique que ya no la necesita.
Si usted tiene dificultades o no entiende las instrucciones consulte a su médico ó farmacéutico.
Pueden pasar varios días antes de que note los beneficios producidos por el medicamento. Es muy importante que lo utilice regularmente cada día. No interrumpa el tratamiento aunque se encuentre mejor a no ser que se lo diga el médico, o note que su respiración empeora cuando toma el medicamento.
No utilice este medicamento para tratar un ataque repentino de dificultad respiratoria, no le ayudará. Necesitará otra clase de medicamento distinto.
Instrucciones para la correcta administración del preparado:
Beclo-Asma 50 microgramos/pulsación se administra por vía inhalatoria. El manejo correcto de este medicamento es decisivo para el éxito del tratamiento.
Antes de cada aplicación se observarán las siguientes pautas:
- Retire la cubierta protectora (fig. 1). En caso de que sea un inhalador nuevo o no se haya utilizado durante una semana, agitar el envase (fig. 2) y efectuar dos pulsaciones para asegurar el buen funcionamiento del inhalador. En caso de que el inhalador se utilice regularmente pase a las instrucciones siguientes:
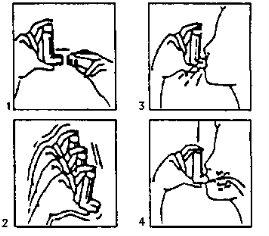 Agite el inhalador
Agite el inhalador- Elimine de sus pulmones la máxima cantidad de aire posible.
- Adapte el inhalador a su boca según la posición que se indica en el dibujo (fig. 3).
- Haga una inspiración lo más profunda posible. Debe oprimir, según las flechas del dibujo (fig. 4), el aparato mientras está haciendo esta inspiración.
- Retire el inhalador de su boca y procure retener el aire en sus pulmones durante unos segundos y expulse el aire lentamente.
- Debe lavarse periódicamente la boquilla de plástico. Para ello, retírela del aerosol y enjuáguelo con abundante agua al menos una vez por semana. Antes de su uso deberá estar seca. No mojar el cartucho metálico.
- Guardar con la cubierta protectora colocada para protegerlo del polvo y de la suciedad.
- Es recomendable enjuagarse la boca con agua después de cada inhalación.
Si estima que la acción de Beclo-Asma 50 microgramos/pulsación es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Si usa más Beclo-Asma 50 microgramos/pulsación del que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico, o llame al Servicio de Información toxicológica, teléfono 915620420, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Beclo-Asma 50 microgramos/pulsación
No tome una dosis doble para compesar las dosis olvidadas, simplemente espere a la siguiente dosis.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos:
-Frecuentes (pueden afectar hasta 1 de cada 10 personas): candidiasis en boca y/o garganta (aftas), ronquera y/o irritación de garganta, alteraciones del gusto.
-Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): dolor de cabeza, vértigo, temblores, tos, síntomas incrementados de asma, náuseas, urticaria, picor, prurito, eritema, púrpura.
-Raros (pueden afectar hasta 1 de cada 1.000 personas): reacciones alérgicas, angioedema en ojos, garganta, labios y cara, broncoespasmo paradójico.
-Muy raros (pueden afectar hasta 1 de cada 10.000 personas): supresión adrenal, retraso del crecimiento en niños y adolescentes, cataratas, glaucoma, disminución de la densidad mineral ósea.
-Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): Hiperactividad psicomotora, trastornos del sueño, ansiedad, depresión, agresividad, cambios en el comportamiento (comúnmente en niños), y visión borrosa.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Beclo-Asma 50 microgramos/pulsación
Mantener este medicamento fuera de la vista y del alcance de los niños.
Proteger de la luz solar directa. No congelar.
Si el inhalador estuviera muy frío, sacar el cartucho y calentar con la mano durante unos pocos minutos antes de usar. No utilizar ningún otro método para calentarlo.
El envase contiene un líquido a presión. No exponer a temperaturas superiores a 50 ºC. No perforar el envase aun cuando aparentemente esté vacío.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de Cad.:. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Beclo-Asma 50 microgramos/pulsación:
- El principio activo es dipropionato de beclometasona.
- Los demás componentes (excipientes) son etanol anhidro y Norflurano.
Cada inhalación contiene 50 microgramos de dipropionato de beclometasona.
Aspecto del producto y contenido del envase:
Beclo-Asma 50 microgramos/pulsación es una solución transparente e incolora que se presenta en un envase con 10 ml (200 aplicaciones), válvula dosificadora y adaptador oral.
Titular de la autorización de comercialización y responsable de la fabricación:
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat
Barcelona – España
Fecha de la última revisión de este prospecto:10/2017
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BECLO-ASMA 50 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 100 microgramos/pulsaciónPrincipio activo: beclometasoneFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 250 µg becaometasona dipropionatoPrincipio activo: beclometasoneFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 50 mcg beclometasona dipropionatoPrincipio activo: beclometasoneFabricante: Glaxosmithkline S.A.Requiere receta
Médicos online para BECLO-ASMA 50 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BECLO-ASMA 50 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















