ATROVENT MONODOSIS 500 mcg/ 2ml SOLUCION PARA INHALACION POR NEBULIZADOR

Cómo usar ATROVENT MONODOSIS 500 mcg/ 2ml SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Atrovent Monodosis 500 microgramos/2 ml solución para inhalación por nebulizador
bromuro de ipratropio
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Atrovent Monodosis y para qué se utiliza
- Qué necesita saber antes de empezar a usar Atrovent Monodosis
- Cómo usar Atrovent Monodosis
- Posibles efectos adversos
- Conservación de Atrovent Monodosis
- Contenido del envase e información adicional
1. Qué es Atrovent Monodosis y para qué se utiliza
Atrovent Monodosis pertenece al grupo de medicamentos denominados broncodilatadores anticolinérgicos, que actúan relajando la musculatura de los bronquios, facilitando así el paso del aire y por tanto, la respiración.
Atrovent Monodosis 500 microgramos/2 ml solución para inhalación por nebulizador es un medicamento que se emplea en el tratamiento del broncoespasmo asociado a enfermedades obstructivas crónicas en adultos y en niños mayores de 12 años.
Atrovent Monodosis puede ser administrado junto con beta-adrenérgicos (otros medicamentos broncodilatadores, como por ejemplo salbutamol) en el tratamiento del espasmo agudo de los bronquios que provoca una obstrucción reversible de las vías aéreas, en aquellos casos en que el tratamiento con un medicamento del grupo de los beta-adrenérgicos no proporcione suficiente dilatación de la musculatura de los bronquios.
2. Qué necesita saber antes de empezar a usar Atrovent Monodosis
No use Atrovent Monodosis
- Si es alérgico (hipersensible) al bromuro de ipratropio, a sustancias que son similares a ipratropio tales como atropina o sus derivados o a cualquiera de los demás componentes de Atrovent Monodosis.
- En episodios de espasmos agudos de los bronquios (dificultad respiratoria) donde se requiera una respuesta rápida.
Advertencias y precacuciones
Consulte a su médico o farmacéutico antes de empezar a usar Atrovent Monodosis:
-Si tiene predisposición a padecer glaucoma de ángulo estrecho (aumento de la presión interna del ojo), presenta hipertrofia prostática (agrandamiento de la próstata) u obstrucción del flujo urinario (dificultad para orinar).
-Se han producido casos aislados de complicaciones en el ojo, como por ejemplo, midriasis (dilatación de la pupila), glaucoma de ángulo estrecho (aumento de la presión interna del ojo) y dolor en el ojo cuando el bromuro de ipratropio, solo o combinado con un agonista ?2 adrenérgico (medicamentos que dilatan la musculatura de los bronquios, como por ejemplo salbutamol), ha penetrado en los ojos por aplicación inadecuada.
-El dolor o malestar del ojo, la visión borrosa, los halos visuales (luces difusas) o imágenes coloreadas en asociación con enrojecimiento de ojos debido a congestión de la conjuntiva y edema (hinchazón) de la córnea pueden ser signos de aumento de la presión interna del ojo (glaucoma de ángulo estrecho). Si aparece alguna combinación de estos síntomas, se debe consultar al médico inmediatamente para iniciar un tratamiento con un colirio de tipo miótico (que produce una contracción de la pupila y disminuye la presión interna del ojo).
-Se debe evitar que el producto contacte con los ojos durante la nebulización, especialmente en aquellos pacientes que tengan predisposición a sufrir un aumento de la presión intraocular, por lo que se recomienda utilizar boquilla o máscara nasofacial.
-Los pacientes con fibrosis quística (una enfermedad que altera las secreciones de las glándulas mucosas y sudoríparas, afectando a varios órganos) pueden ser más propensos a trastornos de la motilidad gastrointestinal.
-En casos excepcionales se pueden producir reacciones de hipersensibilidad inmediata (reacciones alérgicas de rápida aparición) después de la administración de Atrovent Monodosis, tales como urticaria, angioedema (hinchazón repentina de la piel o las mucosas), erupción en la piel, broncoespasmo (contracción de la musculatura de los bronquios que dificulta la respiración) y edema orofaríngeo (hinchazón de la boca y la faringe).
-Como con otros fármacos administrados por vía inhalatoria, Atrovent Monodosis puede causar broncoespasmo paradójico, que puede poner en peligro su vida. En caso de producirse, se debe interrumpir el tratamiento de inmediato e informar al médico para sustituir por un tratamiento alternativo.
Otros medicamentos y Atrovent Monodosis
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se recomienda la administración simultánea por un periodo largo de tiempo de Atrovent Monodosis con otros fármacos anticolinérgicos.
Los beta-adrenérgicos (p. ej. salbutamol) y los derivados de la xantina (p. ej. teofilina) son otros medicamentos broncodilatadores y pueden potenciar el efecto broncodilatador. Atrovent Monodosis puede aumentar los efectos anticolinérgicos de otros fármacos.
Atrovent Monodosis se puede administrar junto con otros fármacos comúnmente utilizados en el tratamiento de la obstrucción reversible de las vías respiratorias, incluyendo medicamentos beta-adrenérgicos (p. ej. salbutamol), metilxantinas (p. ej. teofilina), esteroides y cromoglicato disódico, sin aparición de interacciones que hagan necesario ajustar la dosis.
La administración simultánea de bromuro de ipratropio nebulizado y betamiméticos puede incrementar el riesgo de glaucoma agudo en pacientes con historial de glaucoma de ángulo estrecho (aumento de la presión intraocular).
Las soluciones para inhalar de Atrovent Monodosis y de cromoglicato disódico que contengan cloruro de benzalconio como conservante, no se deben administrar simultáneamente en el mismo nebulizador, por riesgo de precipitación.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
A pesar de que en los estudios preclínicos no se ha demostrado ningún riesgo, no se ha establecido su seguridad durante el embarazo. Por ello, deben observarse las precauciones habituales en el uso de medicamentos en este período, especialmente durante los 3 primeros meses.
Se desconoce si bromuro de ipratropio puede pasar a la leche materna. No obstante es improbable que pueda ser ingerido por el lactante en cantidades significativas, especialmente porque el preparado se administra por vía inhalatoria. Sin embargo, se debe administrar con precaución a las mujeres en período de lactancia.
Conducción y uso de máquinas
No se dispone de estudios de los efectos sobre la capacidad para conducir y utilizar máquinas. Sin embargo, se advierte que pueden aparecer efectos adversos como mareos, dificultades del ojo para enfocar, dilatación de las pupilas y visión borrosa durante el tratamiento con Atrovent. Por tanto, se recomienda precaución en la conducción y el uso de máquinas.
3. Cómo usar Atrovent Monodosis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde usar su medicamento.
Su médico determinará la duración del tratamiento.
Si considera que la acción de Atrovent Monodosis es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
La solución de Atrovent Monodosis puede inhalarse (aspirarse) utilizando dispositivos llamados nebulizadores de tipo ultrasónico, eléctrico, manual (por ej. Bird, De Vilbiss, Pari), o con respiración asistida a presión positiva intermitente. Si se dispone de suministro de oxígeno en la pared, debe administrarse la solución con un flujo de 6-8 litros por minuto.
Se recomienda que el tamaño de partícula de la solución nebulizada esté comprendido entre 1 y 10 micras aunque aproximadamente el 50% de la masa total del aerosol debe estar contenido en partículas inferiores a 5 micras.
En caso necesario la solución puede diluirse en suero fisiológico.
Normas para el uso de los envases monodosis
Los envases monodosis deben ser utilizados solamente para su inhalación con dispositivos nebulizadores adecuados y no deben administrarse por vía oral.
|
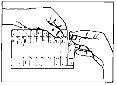
depósito del nebulizador (véase fig. 3).
|
Como los envases monodosis no contienen conservantes es importante que su contenido se utilice inmediatamente después de la apertura del envase, para evitar la contaminación. Deben tirarse los envases monodosis parcialmente utilizados, abiertos o dañados.
La administración de la solución Atrovent Monodosis se debe adaptar a las necesidades individuales de cada paciente; los pacientes deben estar bajo supervisión médica durante el tratamiento. Se aconseja no rebasar la dosis diaria recomendada tanto para el tratamiento agudo como para el de mantenimiento. Las dosis recomendadas son:
Adultos y niños mayores de 12 años
Tratamiento de mantenimiento
1 envase monodosis, 3-4 veces al día.
Ataques agudos
En aquellos casos en que sólo se utiliza un medicamento beta-adrenérgico puede que éste no proporcione suficiente dilatación de la musculatura de los bronquios. En estos casos, Atrovent Monodosis puede ser administrado en asociación con un beta-adrenérgico inhalado (p. ej. salbutamol), cuya dosis será establecida por el médico.
La dosis de Atrovent Monodosis, en este caso, es de un envase monodosis; se pueden administrar dosis repetidas hasta que el paciente se estabilice.
Por lo general, no debe superarse la dosis diaria recomendada durante el tratamiento. Las dosis diarias de más de 2 mg (más de 4 envases monodosis) deben administrarse exclusivamente bajo supervisión médica.
Debe consultar a su médico siempre que no consiga una mejoría significativa o que su estado empeore, con el fin de determinar un nuevo tratamiento. En caso de disnea aguda o de disnea que se agrave rápidamente (dificultades respiratorias), hay que consultar al médico inmediatamente.
Si usa más Atrovent Monodosis del que debe
No se han descrito manifestaciones específicas de sobredosis. Debido al amplio margen terapéutico de Atrovent Monodosis y a la administración inhalatoria del preparado, no es de esperar la aparición de síntomas anticolinérgicos serios.
En caso de producirse síntomas anticolinérgicos menores como sequedad de boca, trastornos de la acomodación visual (problemas del ojo para enfocar) y taquicardia (aumento de la frecuencia cardiaca), el tratamiento a seguir debería ser para aliviar los síntomas.
Si usted ha utilizado más Atrovent Monodosis de lo que debe, consulte a su médico, farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó usar Atrovent Monodosis
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos frecuentes (se presentan en al menos 1 de cada 100 pacientes) son dolor de cabeza (cefalea), mareo, tos, irritación de garganta, náuseas, sequedad de boca, trastornos de la motilidad gastrointestinal (por ejemplo: cambio en el hábito intestinal, reflujo gastroesofágico, dispepsia (indigestión)).
Los efectos adversos poco frecuentes (se presentan en al menos 1 de cada 1.000 pacientes) son
hipersensibilidad, reacción anafiláctica (reacción alérgica grave), visión borrosa, midriasis (dilatación de la pupila), aumento de la presión interna del ojo, halos visuales (luces difusas) o imágenes coloreadas asociadas a enrojecimiento de los ojos (glaucoma), dolor ocular, halos visuales (luces difusas), enrojecimiento de los ojos, edema de córnea (hinchazón de la córnea), palpitaciones, taquicardia supraventricular, estreñimiento, diarrea, vómitos, estomatitis (inflamación de la boca), edema bucal (hinchazón de la boca), erupción, prurito (picor), angioedema (hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad para tragar o respirar) y retención de orina.
Los efectos adversos raros (se presentan en al menos 1 de cada 10.000 pacientes) son broncoespasmo (opresión en el pecho, pitos o falta de respiración), broncoespasmo paradójico (estrechamiento de las paredes de los bronquios debido a la propia inhalación), contracción de laringe, edema faríngeo (hinchazón de garganta), sequedad de garganta, trastorno de la acomodación visual (dificultad del ojo para enfocar), urticaria, aumento de la frecuencia cardíaca y fibrilación auricular.
Los efectos adversos muy raros (se presentan en menos 1 de cada 10.000 pacientes) son temblores, sabor metálico o desagradable, congestión nasal, insomnio, cansancio o debilidad no habitual e hipotensión.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Atrovent Monodosis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30ºC. Conservar en el embalaje original.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Atrovent Monodosis
- El principio activo es bromuro de ipratropio. Cada envase monodosis de 2 ml contiene 522 microgramos de bromuro de ipratropio monohidratado (equivalente a 500 microgramos de bromuro de ipratropio anhidro).
- Los demás componentes son: cloruro sódico, ácido clorhídrico y agua purificada.
Aspecto del producto y contenido del envase
Atrovent Monodosis 500 microgramos/2 ml es una solución para inhalación por nebulizador que se presenta en cajas de 20 envases monodosis con 2 ml de solución para inhalación por nebulizador y en envase clínico con 100 envases monodosis de 2 ml de solución para inhalación por nebulizador.
Titular de la autorización de comercialización
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
España
Responsable de la fabricación
Laboratoire Unither
Zone Industrialle de Longpré
10 rue Andre Durouchez
80084 AMIENS Cedex 2
Francia
Este prospecto ha sido aprobado en Abril 2019
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ATROVENT MONODOSIS 500 mcg/ 2ml SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INHALACIÓN PULMONAR, 0,0040 g/aerosolPrincipio activo: Ipratropio bromuroFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 20 µgPrincipio activo: Ipratropio bromuroFabricante: Boehringer Ingelheim Espana S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 250 µgPrincipio activo: Ipratropio bromuroFabricante: Boehringer Ingelheim Espana S.A.Requiere receta
Médicos online para ATROVENT MONODOSIS 500 mcg/ 2ml SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ATROVENT MONODOSIS 500 mcg/ 2ml SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes