
ASMANEX TWISTHALER 400 micrograms INHALATION POWDER


How to use ASMANEX TWISTHALER 400 micrograms INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Asmanex Twisthaler 400 micrograms inhalation powder
mometasone furoate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Asmanex Twisthaler is and what it is used for
- What you need to know before you use Asmanex Twisthaler
- How to use Asmanex Twisthaler
- Possible side effects
- Storing Asmanex Twisthaler
- Contents of the pack and other information
1. What Asmanex Twisthaler is and what it is used for
Asmanex Twisthaler contains the active substance mometasone furoate, which belongs to a group of medicines called corticosteroids or simply steroids.
Corticosteroids should not be confused with “anabolic” steroids, which are used improperly by some athletes. Corticosteroids are used to prevent asthma attacks as they have an anti-inflammatory effect. They reduce inflammation and irritation of the walls of the small airways in the lungs. This helps to make breathing easier.
Asmanex Twisthaler is used regularly to control asthma in adults and adolescents from 12 years of age. It can be given if you have not used a corticosteroid before and your asthma is not controlled with other medicines. It can also be used to control your asthma if you have been taking a different corticosteroid by mouth or by inhalation. Used regularly, Asmanex Twisthaler will help you control your asthma and prevent asthma attacks. It is a “preventive” medicine. Do not use it in a sudden asthma attack. You should always carry the bronchodilator (“reliever” inhaler) that your doctor has prescribed, just in case you have an asthma attack.
2. What you need to know before you use Asmanex Twisthaler
Do not use Asmanex Twisthaler
- if you are allergic to mometasone furoate, to any other corticosteroid or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor or pharmacist before you start using Asmanex Twisthaler if:
- you have or have had tuberculosis.
- you have an eye infection by herpes simplex (virus) or any other type of infection.
- you have thrush (white patches in your mouth or throat) as you may need treatment. Your doctor may also tell you to stop using Asmanex Twisthaler for a while or may decide to treat you with a different type of inhaler. Rinse your mouth with water or with a mouthwash and then spit it out without swallowing after taking your dose; this will help prevent thrush.
Tell your doctor if you have blurred vision or other visual disturbances.
Important things to remember when using Asmanex Twisthaler
- Seek medical help immediately if you start to wheeze or have difficulty breathing shortly after using Asmanex. These symptoms may be an “allergic” reaction to this medicine.
- You should always carry a bronchodilator (a “reliever” inhaler) to quickly relieve asthma symptoms (such as coughing, wheezing and tightness of the chest).
- If you have an asthma attack and it does not improve after using the “reliever” inhaler or if your peak flow measurement falls (your doctor will tell you what the correct values are for you), you should seek medical help immediately. Tell your doctor, as your asthma may be getting worse and you may need to change your medicine.
- You may also need to take a corticosteroid in tablet or syrup form during a severe asthma attack, during other illnesses or during periods of stress. Your doctor may prescribe a corticosteroid in tablet or syrup form for you to carry with you and may also give you a steroid treatment card, which will tell you when and how to use it.
- If you are taking Asmanex and reduce the dose of oral corticosteroids or syrup, you may start to have symptoms such as itchy eyes, tearfulness or skin rash, which were previously controlled. Your doctor will tell you how to control these symptoms. During this time, if you start to feel joint or muscle pain, feeling depressed, tired or sluggish, you should tell your doctor.
- While using inhaled corticosteroids, avoid contact with anyone who has measles or chickenpox. If you come into contact with anyone who has one of these illnesses, tell your doctor. This is especially important in children.
- Treatment with medicines like Asmanex Twisthaler may affect the normal levels of corticosteroids in your body. One effect of this may be that adolescents being treated for a long time may grow more slowly. Your doctor will probably check your height from time to time.
- All corticosteroids, especially when used for a long time, may affect the functioning of the adrenal gland. Your doctor may do a blood test to check this.
- Remember to take this medicine and any other medicine you are taking with you if you have to go to the hospital.
- Your doctor may do lung tests during your treatment, especially if you are stopping oral corticosteroids.
Children and adolescents
There are no data available, and therefore it should not be used in children under 12 years of age.
Other medicines and Asmanex Twisthaler
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription. This includes medicines for treating a fungal or viral infection, such as ketoconazole, itraconazole, ritonavir, nelfinavir or cobicistat. These medicines may increase the amount of corticosteroid in your blood.
Some medicines may increase the effects of Asmanex Twisthaler, so your doctor will monitor you closely if you are taking these medicines.
It is also important that you tell your doctor before you start using Asmanex Twisthaler if you are taking other corticosteroids, by injection, orally or by inhalation. Your doctor will tell you how to adjust the dose of these other corticosteroids.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Asmanex Twisthaler does not affect your ability to drive or use machines.
Asmanex Twisthaler contains lactose
It may cause allergic reactions in patients with cow's milk protein allergy.
If your doctor has told you that you have an intolerance to some sugars, contact them before using this medicine.
This medicine contains a small amount of lactose (4.64 mg/day in the maximum recommended daily dose). This amount normally does not cause problems in people with lactose intolerance.
3. How to use Asmanex Twisthaler
Make sure you know how to use the inhaler properly. Avoid dropping the inhaler, as it may break. If you have any problems, consult your doctor or pharmacist. Make sure you know when and how many doses to inhale. Your doctor should have told you and the instructions are in the package leaflet. Follow your doctor's or pharmacist's instructions for using this medicine exactly. Do not change the dose unless your doctor tells you to.If in doubt, consult your doctor or pharmacist again.
For adults (including those over 65 years of age) and adolescents over 12 years of age with mild to moderate asthma, the recommended initial dose is 400 micrograms (1 dose or inhalation) once daily in the evening, using Asmanex Twisthaler 400 micrograms inhalation powder. However, for some patients the initial dose may be 200 micrograms (1 dose or inhalation) twice daily, using Asmanex Twisthaler 200 micrograms inhalation powder. If your asthma is controlled, your doctor may reduce the dose to 200 micrograms (1 dose or inhalation of Asmanex Twisthaler 200 micrograms inhalation powder) once daily in the evening. Depending on your symptoms, your doctor may increase or decrease your daily dose to keep your asthma under control.
For patients with severe asthma who may be taking other corticosteroids, in tablet or syrup form, the usual initial dose of Asmanex Twisthaler is 400 micrograms twice daily, which is the highest recommended dose. After about one week of treatment with Asmanex Twisthaler, your doctor may start to slowly reduce the dose of the other corticosteroid and eventually stop it altogether. Then, if your asthma is well controlled, it may be possible to reduce the dose of Asmanex Twisthaler.
Instructions for proper use of the inhaler
Remember that Asmanex Twisthaler produces a very fine powder that you must inhale into your lungs. You should be standing when using your inhaler. To get the correct dose, follow these instructions carefully.
Before removing the white cap, make sure the counter and the arrow on the cap are aligned. To open the inhaler, remove the white cap. Hold the inhaler in an upright position, with the purple base down. Hold it by the base and turn the cap counterclockwise to remove it. When you remove the cap, the dose counter on the inhaler will decrease by one unit.
Make sure the counter on the purple base and the arrow on the counter are aligned with each other. Hold the inhaler upright once the cap is removed and before inhaling your dose.
To inhale your dose:
- Remove the cap from the inhaler. [Figure 1]
- Bring the inhaler to your mouth, with the mouthpiece facing you.
- Place the mouthpiece of the inhaler in your mouth, close your lips around the mouthpiece, and inhale quickly and deeply. [Figure 2]
- Remove the inhaler from your mouth and hold your breath for about 10 seconds or as long as you comfortably can. Never breathe out through the inhaler.
- To close the inhaler, replace the cap immediately after each inhalation. The cap must be fully closed and turned clockwise while pressing down gently until you hear a “click” that indicates the cap is fully closed. [Figure 3]. The arrow on the cap should be fully aligned with the counter window. [Figure 4].
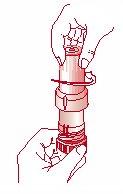
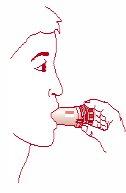
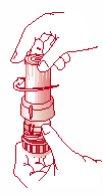
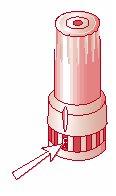
Fig. 1 Fig. 2 Fig. 3 Fig. 4
Remove the cap Inhalation Close the inhaler Inhaler closed
Once you have finished taking your dose, rinse your mouth with water or with a mouthwash and then spit it out without swallowing. This will help prevent thrush.
Keep your inhaler clean and dry at all times. Clean the outside of the mouthpiece with a dry cloth or tissue. Do not wash the inhaler; avoid contact with water.
On the base of the inhaler, there is a window with a counter that shows the number of doses left in the inhaler. Do not use Asmanex Twisthaler if you notice that the counter is not working correctly. Take it to your doctor or pharmacist.
When “01” appears in the counter window, it means that there is only one dose left in the inhaler. After inhaling the dose 01, the counter will show “00”, the cap will be locked, and no new doses can be obtained from the inhaler. In this situation, discard the inhaler.
To ensure that you always have enough medication, ask your doctor to prescribe a new one before the inhaler you are using runs out.
The time to relief of symptoms varies between individuals. Although some patients experience an improvement within 24 hours of starting treatment, others may not obtain the maximum benefit until one to two weeks or more. Your doctor will tell you how long you should continue using Asmanex Twisthaler.
If you use more Asmanex Twisthaler than you should
It is important that you take your dose as indicated on the label or as advised by your doctor.
Do not increase or decrease your dose without medical supervision.
If you accidentally take a higher dose than prescribed, tell your doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, stating the medicine and the amount taken. Telephone (91) 562 04 20.
If you forget to use Asmanex Twisthaler
If you forget a dose, simply take the next dose when it is due. Do not take a double dose to make up for forgotten doses.
If you stop using Asmanex Twisthaler
Do not stop using Asmanex Twisthaler suddenly, even if your asthma seems to be getting better. First talk to your doctor.
Your symptoms may come back if you stop using this medicine before your doctor tells you to. If you think that your asthma is not getting better or is getting worse after you start using Asmanex Twisthaler, go back to see your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Asmanex Twisthaler and tell your doctor immediately if you notice any of the following symptoms:
Symptoms of a severe allergic reaction including swelling of the eyes, face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing, skin rash with itching, feeling faint or dizzy and fainting which may cause fainting.
If you notice an increase in coughing, wheezing, difficulty breathing or shortness of breath immediately after inhaling the medicine, use your “reliever” inhaler and contact your doctor immediately. Do not use Asmanex Twisthaler again until you have seen your doctor.
Allergic reactions (hypersensitivity) to inhaled corticosteroids can occur. If this happens, you may notice a skin rash, itching and redness and swelling of the eyes, face, lips and throat. If you get any of these symptoms, tell your doctor immediately. Do not use Asmanex Twisthaler again until you have seen your doctor.
The most frequently reported side effects include infection with Candida (thrush) in the mouth or throat (white patches), hoarseness, sore throat or headache. Contact your doctor as soon as possible if you develop any of these side effects.
Other uncommon side effects (affecting less than 1 in 100 patients) may include dryness of the mouth and throat, indigestion, weight gain and rapid or strong heartbeat (palpitations). Contact your doctor if you notice any of these symptoms.
In rare cases (affecting less than 1 in 1,000 patients), patients taking inhaled corticosteroids, including Asmanex Twisthaler, have developed increased eye pressure (including glaucoma) or cataracts.
Frequency not known (cannot be estimated from the available data): blurred vision.
Contact your doctor if you have blurred vision or eye pain.
Other possible side effects of corticosteroids are a slowing or alteration of growth rate in adolescents and a decrease in bone mineral density. They can also cause sleep problems, depression or feeling worried, anxious, nervous, excited or irritable with the use of inhaled corticosteroids. Children are more likely to experience these effects. Corticosteroids can also affect the functioning of your adrenal gland, causing weakness, tiredness or dizziness, as well as fainting when standing for a long time or when getting up from a sitting position. It is much less likely that these effects will occur with the use of inhaled corticosteroids than with the use of oral corticosteroids.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible that they are not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Asmanex Twisthaler
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box, aluminum bag, and label, after EXP. The expiration date is the last day of the indicated month.
Do not store at a temperature above 30°C. Do not refrigerate or freeze.
Keep each inhaler in its original packaging to protect it from moisture until you need it, and then use it within 3 months of opening. Open only one inhaler at a time.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and unused medicines at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Asmanex Twisthaler
- The active ingredient is mometasone furoate. Each inhalation contains 400 micrograms.
- The other component is anhydrous lactose (which contains traces of milk proteins).
Appearance of the Product and Package Contents
The mouthpiece of the inhaler is white, with a purple base that contains a window showing the number of inhalations remaining in the inhaler. There are inhalers of 14, 30, or 60 inhalations (doses), each package contains a bag with an inhaler (for each 14, 30, or 60 inhalations) or three individual bags, each with an inhaler (only for 60 inhalations).
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Organon Health, S.L.
Paseo de la Castellana, 77
28046 Madrid
Spain
Tel.: 915911279
Manufacturer
Organon Heist bv
Industriepark 30
2220 Heist-op-den-Berg
Belgium
This medication is authorized in the Member States of the European Economic Area under the following names:
Germany: Asmanex Twisthaler 400 Mikrogramm Pulver zur Inhalation
Austria: Asmanex Twisthaler
Belgium: Asmanex Twisthaler 400 microgrammes Poudre pour inhalation
Denmark: Asmanex Twisthaler 400 mikrogram inhalationspulver
Spain: Asmanex Twisthaler 400 microgramos polvo para inhalación
Finland: Asmanex Twisthaler
France: Asmanex Twisthaler 400 microgrammes/dose, poudre pour inhalation
Greece: Asmanex Twisthaler
Ireland: Asmanex Twisthaler 400 micrograms Inhalation Powder
Iceland: Asmanex Twisthaler, 400 míkróg. Innöndunarduft
Italy: Asmanex 400 microgrammi polvere per inalazione
Norway: Asmanex Twisthaler
Portugal: Asmanex Twisthaler
United Kingdom: Asmanex Twisthaler 400 micrograms Inhalation Powder
Sweden: Asmanex Twisthaler
Date of the last revision of this leaflet: 11/2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price51.14 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ASMANEX TWISTHALER 400 micrograms INHALATION POWDERDosage form: PULMONARY INHALATION, 200 mcgActive substance: mometasoneManufacturer: Organon Salud S.L.Prescription requiredDosage form: PULMONARY INHALATION, 160 MCGActive substance: ciclesonideManufacturer: Covis Pharma Europe B.V.Prescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for ASMANEX TWISTHALER 400 micrograms INHALATION POWDER
Discuss questions about ASMANEX TWISTHALER 400 micrograms INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















