
ALVESCO 160 microgramos/INHALACION SOLUCION PARA INHALACION EN ENVASE A PRESION

Cómo usar ALVESCO 160 microgramos/INHALACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Alvesco 160 microgramos/inhalación, solución para inhalación en envase a presión
Ciclesonida
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Alvesco y para qué se utiliza
- Qué necesita saber antes de empezar a usar Alvesco
- Cómo usar Alvesco
- Posibles efectos adversos
- Conservación de Alvesco
- Contenido del envase e información adicional
1. Qué es Alvesco y para qué se utiliza
¿Qué es Alvesco?
Alvesco es un aerosol transparente e incoloro para ser inhalado por la boca hasta los pulmones. Es un medicamento preventivo (corticosteroide) que debe tomarse a diario y que sólo es activo cuando se inhala hasta los pulmones.
La sustancia activa en este medicamento es la Ciclesonida (Para consultar otros componentesver sección 6).
¿Para qué se utiliza Alvesco?
Alvesco se usa para tratar el asma persistente en adultos y adolescentes de 12 años en adelante.
¿Cómo actúa Alvesco?
Alvesco le ayuda a respirar mejor, disminuyendo los síntomas del asma y la probabilidad de sufrir una crisis asmática. El efecto del medicamento se potencia con el tiempo y, por este motivo, debe tomarse a diario, incluso cuando se sienta bien.
Este medicamento no es adecuado para las crisis de asma. Utilice su inhalador de rescate para aliviar rápidamente estas crisis.
2. Qué necesita saber antes de empezar a usar Alvesco
No use Alvesco
si es alérgico a ciclesonida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Alvesco.
Tenga especial cuidado con Alvesco
- Antes de iniciar el tratamientocon este medicamento, informe a su médico si:
Recibió o está recibiendo algún tratamiento frente a la tuberculosis pulmonar e infecciones fúngicas, víricas o bacterianas.
Consulte a su médico, si tiene dudas. Es importante asegurarse de que Alvesco es el medicamento adecuado para usted.
Durante el tratamiento con Alvesco, acuda inmediatamentea su médico si:
- comienza a tener dificultad para respirar y empeoran algunos de sus síntomas: tos, asfixia, sibilancia, opresión en el pecho, ruidos (roncus) u otros síntomas de constricción de las vías aéreas. (Debe usar su inhalador de rescate, lo que normalmente le producirá una rápida mejoría).
- si se despierta por la noche con los síntomas.
- si no mejora a pesar de usar el inhalador de rescate.
Su médico decidirá sobre la continuación con el tratamiento.
Si presenta visión borrosa u otros problemas visuales, póngase en contacto con su médico.
Grupos especiales de pacientes
Pacientes con asma gravetienen el riesgo de sufrir crisis asmáticas. Para estos pacientes, el médico controlará regularmente el asma, e incluso realizará una prueba de la función pulmonar.
Pacientes que estén tomando corticosteroides en comprimidos:
Alvesco puede usarse para sustituir a los corticosteroides en comprimidos o para reducir el número de tomas de los mismos. Siga con atención las indicaciones de su médico.
- Esta situación se podrá realizar tras aproximadamente 1 semana después de iniciar las inhalaciones con Alvesco.
- El número de comprimidos se irán reduciendo con precaución durante un periodo de tiempo.
- Durante este periodo, puede que a veces sufra una sensación general de malestar.
- Aun así, es conveniente que siga inhalando Alvesco y reduciendo progresivamente el número de comprimidos.
- Si sufre síntomas graves como náuseas, vómitos, diarrea o fiebre, acuda a su médico.
- Este proceso puede dar lugar a reacciones alérgicas menores como rinitis (inflamación la nariz) o eccema (picor y enrojecimiento de la piel).
- Si ha cambiado el tratamiento de comprimidos, seguirá expuesto durante un tiempo al riesgo de sufrir insuficiencia suprarrenal. Dicho riesgo está relacionado con la ingesta de corticosteroides en comprimidos. Los síntomas de la insuficiencia suprarrenal (mareo, desmayo, náusea, pérdida del apetito, cambios de humor, caída del vello corporal, incapacidad de superar situaciones de estrés, debilidad, dolores de cabeza, problemas de memoria, alergias, ansiedad por comer y trastornos de la glucosa en sangre) pueden persistir durante un tiempo.
- Puede tener que acudir a un especialista para que le determine el grado de reducción de la función suprarrenal.
- Su médico además realizará controles periódicos de la función suprarrenal.
- Durante los periodos de estrés, por ejemplo, intervenciones quirúrgicas o empeoramientos de las crisis asmáticas, es probable que necesite tomar algún corticosteroide en comprimidos adicional. De ser así, debe portar una tarjeta identificativa que indique dicha ingesta.
Pacientes con trastornos hepáticos o renales
Si padece trastornos hepáticos o renales, no es necesario realizar el ajuste de dosis de ciclesonida.
Si sufre algún trastorno hepático grave, su médico le examinará con más detenimiento para ver si existen efectos secundarios consecuencia de una alteración en la producción normal de esteroides.
Niñosmenores de 12 años:
Este medicamento no está recomendado para niños menores de 12años debido a la falta de información sobre sus posibles efectos secundarios.
Otros medicamentos y Alvesco
Informe a su médico antes de usar Alvesco, si está recibiendo tratamiento contra una infección fúngica o vírica con medicamentos que contienen:
- ketoconazol
- itraconazol
- ritonavir
- nelfinavir
Estos compuestos pueden aumentar el efecto de Alvesco de forma que no se pueda evitar por completo la aparición de efectos secundarios.
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Uso de Alvesco con los alimentos y bebidas
Alvesco se puede tomar con o sin bebida y comida.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
- Su médico le informará de los riesgos y beneficios de usar Alvesco, ya que no existen suficientes datos sobre los efectos de Alvesco en embarazadas.
- Alvesco puede utilizarse durante el embarazo solo cuando los beneficios posibles para la madre superen los posibles riesgos para el feto. Si su médico decide que puede seguir usando Alvesco, debe utilizar la dosis mínima de ciclesonida con la que se mantenga el control del asma.
- En niños de madres tratadas durante el embarazo con corticosteroides se comprobará detenidamente la función suprarrenal.
- Consulte a su médico si desea usar Alvesco durante la lactancia.
- Se desconoce si la ciclesonida inhalada pasa a la leche materna.
- El uso de Alvesco en mujeres en periodo de lactancia sólo se considerará si los beneficios previstos para la madre superan los posibles riesgos para el niño.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
No se conocen efectos de Alvesco sobre la capacidad de conducir y utilizar máquinas.
Alvesco contiene etanol
Este medicamento contiene 4,7 mg de alcohol (etanol) en cada dosis. La cantidad en una dosis de este medicamento es equivalente a menos de 1 ml de cerveza o vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible.
3. Cómo usar Alvesco
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- Si acaba de empezar a usar este medicamento en lugar o a la vez que corticosteroides en comprimidos, ver sección 2, Pacientes que estén tomando corticosteroides en comprimidos.
¿Cuánto Alvesco debo usar diariamente?
Su médico le habrá informado de la cantidad de medicamento que necesita usar diariamente. Dicha cantidad se establecerá en función de sus necesidades.
- La dosis recomendada de Alvesco es 160 microgramos diarios en dosis única. Esta dosis alivia el asma en la mayoría de los pacientes.
- En algunos pacientes una reducción de la dosis a 80 microgramos diarios en una única dosis puede ser adecuada para mantener un control eficaz del asma.
- En algunos pacientes con empeoramiento grave de los síntomas del asma puede ser necesario aumentar la dosis de Alvesco durante un breve periodo de tiempo. Esta dosis puede llegar hasta 640 microgramos diarios, divididos en dos dosis de 320 microgramos cada una, aunque no se dispone de datos que confirmen el efecto terapéutico adicional tras tres meses de tratamiento con esta dosis.
Si fuera necesario, su médico puede recetarle corticosteroides en comprimidos o, en el caso de que exista alguna infección, un antibiótico.
- Su médico ajustará su dosis a la mínima necesaria para controlar el asma.
- Debe comenzar a notar una mejora de los síntomas (silbido al respirar, opresión en el pecho y tos) en el plazo de 24 horas.
¿Cuándo debo usar mi inhalador Alvesco?
En la mayoría de los casos, por la mañana o por la tarde, en una o dos inhalaciones una vez al día. Siga con mucha atención las indicaciones de su médico. Es importante que use Alvesco regularmente todos los días, incluso cuando se encuentre mejor.
Si nota que debe usar su inhalador de rescate más de dos o tres veces a la semana, debe acudir a su médico para que le revise su tratamiento.
¿Cómo uso el inhalador Alvesco?
Es importante que un médico, enfermero o farmacéutico le enseñe en primer lugar cómo usar correctamente el inhalador. Una buena técnica garantizará que la cantidad de Alvesco que llega a sus pulmones sea la adecuada. Siga las instrucciones de este prospecto para recordar su uso.
Es conveniente practicar delante del espejo las dos primeras veces hasta estar seguro de que utiliza correctamente el inhalador de Alvesco. Debeasegurarse de que ninguna cantidad de medicamento se pierde por la parte superior o los laterales de la boca.
Si tiene un inhalador nuevo, o si no ha usado su inhalador durante una semana o más, tiene queprobarlo antes de su uso. Retire la tapa de la boquilla y, con el inhalador alejado, pulse tresveces el cartucho dentro del inhalador para realizar tresdescargas al aire.
Noes necesario agitar el inhalador de Alvesco antes de usarlo. El medicamento se encuentra en una solución adecuada que garantiza la recepción de la dosis correcta con cada inhalación.
Durante la inhalación, puede permanecer sentado o de pie.
Siga con atención estas instrucciones y utilice las imágenes para guiarse.
- Retire la tapa de la boquilla y compruebe la boquilla, por dentro y por fuera, para asegurarse de que está limpia y seca.

- Sostenga el inhalador al revés (la base del cartucho hacia arriba) con el dedo índice en la base del cartucho y el pulgar debajo de la boquilla.

- Expulseel aire de sus pulmones lo más que pueda. No expulse el aire dentro del inhalador.
- Coloque la boquilla en su boca y cierre los labios firmementealrededor de esta.

- Inmediatamente después de comenzar a aspirar por la boca, pulse con el dedo índice en la parte superior del inhalador para hacer una descarga mientras sigue aspirando lenta y profundamente. Tenga cuidado de que en la descarga no se escape medicamento por la parte superior o laterales de la boca.
- Contenga la respiración, retire el inhalador de la boca y el dedo índice de la parte superior del inhalador. Continúe aguantando la respiración durante aproximadamente 10 segundos o hasta que empiece a sentirse incómodo aguantando la respiración. Espire lentamente por la boca, pero no expulse el aire dentro del inhalador.
Es conveniente no apresurarse en los pasos del 3 al 6

- Si se le ha indicado que realice otra descarga, espere aproximadamente medio minuto y repita los pasos del 3 al 6.
- Después de su uso, coloque siempre la tapa de la boquilla para evitar que entre polvo. Al taparlo bien se escuchará un chasquido, que indica que se ha cerrado correctamente.

- Por motivos de higiene
- Limpie semanalmente la boquilla con un pañuelo seco por dentro y por fuera.
- Use un pañuelo seco y doblado, y limpie la parte frontal del pequeño orificio por el que sale el medicamento.
- Noutilice agua ni otros líquidos.
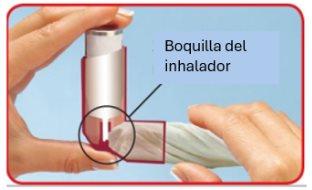
Una buena técnica garantizará que la cantidad de medicamento que llegue a sus pulmones sea la adecuada cada vez que utilice el inhalador. Su médico comprobará que utiliza una técnica adecuada de inhalación para asegurarse de que el tratamiento pueda tener su mayor efecto.
Cuando el cartucho esté completamente vacío, no se escuchará o sentirá la descarga del gas propulsor.
Si comienza a tener dificultades para respirar o siente una opresión en el pecho después de usar el inhalador Alvesco:
- no realice más descargas.
- use su inhalador de rescate para ayudarle a respirar.
- informe inmediatamente a su médico.
Si tiene dificultades para usar el inhalador, su médico podrá recomendarle el uso de una cámara de inhalación. La cámara de inhalación compatible con el inhalador Alvesco se denomina AeroChamber Plus. Si utiliza el dispositivo AeroChamber Plus, siga sus instrucciones de uso. Su médico o farmacéutico podrá asesorarle sobre el uso de este dispositivo.
Si usa más Alvesco del que debiera
Es importante que tome su dosis según las indicaciones de su médico. No debe aumentar o disminuir su dosis sin consultarlo con su médico.
No es necesario aplicar ningún tratamiento específico en el caso de usar más Alvesco del que debiera, aunque deberá informar a su médico. Si usa dosis elevadas durante largos periodos de tiempo, no podrá descartarse la aparición de cierto grado de reducción de la función suprarrenal y puede que sea necesario controlar la función suprarrenal.
Si olvidó usar Alvesco
Si olvida usar el inhalador Alvesco, use la dosis habitual en la próxima toma. Norealice una doble descarga de la dosis habitual.
Si interrumpe el tratamiento con Alvesco
Aunque se sienta mejor no debe dejar de usar su inhalador Alvesco.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si deja de usar Alvesco, debe informar inmediatamente a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
- reacciones alérgicas graves como: hinchazón de los labios, lengua y garganta (pueden afectar a 1de cada 1.000 pacientes tratados)
- reacciones alérgicas: sarpullido en la piel, enrojecimiento, picor o ronchas tipo urticaria (pueden afectar hasta a 1 a 1000 pacientes tratados 1 entre 100 pacientes)
- La tos o sonido sibilante pueden empeorar tras una inhalación (pueden afectar a 1 de cada 100 pacientes tratados)
El resto de los efectos adversos observados con Alvesco son normalmente leves. En la mayoría de los casos se puede continuar con el tratamiento. Los efectos adversos que puede padecer son:
Efectos poco frecuentes (pueden afectar hasta a 1 de cada 100 pacientes tratados):
- ronquera,
- ardor, inflamación, irritación de la boca o garganta
- aftas bucales (infecciones por hongos en la cavidad bucal)
- dolor de cabeza
- mal sabor de boca
- sequedad de la boca o garganta
- náuseas o vómitos
Efectos adversos raros (pueden afectar hasta a 1 de cada 10.000 pacientes tratados):
- sensación de latidos del corazón (palpitaciones)
- molestias o dolor en el abdomen.
- tensión arterial elevada
Efectos adversos de frecuencia no conocida, pero que también pueden ocurrir:
- trastornos del sueño, depresión o sentirse preocupado, inquieto, nervioso, sobre-excitado o irritable. Estos efectos son más probables en niños
- visión borrosa
Alvesco puede afectar a la producción normal de corticosteroides de su cuerpo. Esto normalmente se detecta en pacientes que toman dosis elevadas durante un largo periodo de tiempo. Entre estos efectos poco frecuentes se encuentran:
- retraso en el crecimiento en adolescentes
- pérdida de densidad ósea
- posible opacidad del cristalino (cataratas) que causa una visión borrosa
- pérdida de visión causada por una presión ocular excesivamente alta (glaucoma)
- cara de luna llena, ganancia de peso en el tronco y adelgazamiento de brazos y piernas (cuadro cushingmoideo ó síndrome de Cushing).
El médico debe revisar regularmente la altura de los adolescentes tratados durante un largo periodo de tiempo. Si el ritmo de crecimiento baja, su médico le ajustará el tratamiento, si es posible a la dosis más baja capaz de mantener el control efectivo del asma.
Los corticosteroides en comprimidos pueden provocar más efectos adversos que los inhalados como Alvesco. Si ha tomado corticosteroides en comprimidos antes o durante el tratamiento con Alvesco, el riesgo de sufrir efectos adversos relacionados con la ingesta de este tipo de comprimidos puede persistir durante un tiempo. Si realiza revisiones regulares, su médico deberá comprobar si está tomando la dosis adecuada de Alvesco. En estas consultas se podrán identificar efectos adversos en fase temprana y reducir así las posibilidades de que se agraven.
Recuerde que:
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es . Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Alvesco
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, después de EXP. La fecha de caducidad es el último día del mes que se indica.
El envase contiene un líquido a presión. No conservar a temperatura superior a 50ºC.
No perfore, rompa o queme el envase ni siquiera cuando esté aparentemente vacío.
Al igual que todos los medicamentos para inhalar en envases a presión, el efecto terapéutico de este medicamento puede disminuir si el envase está frío. No obstante, Alvesco libera una dosis constante en un rango de temperaturas comprendido entre 10 ºC bajo cero y 40 ºC.
Si su médico decide interrumpir el tratamiento o si se agota el inhalador, devuélvalo al farmacéutico para que pueda eliminarlo de forma segura. Esto es importante porque pueden quedar restos de medicamento en el envase, aunque parezca que está totalmente vacío. No perfore, rompa o queme el envase, ni siquiera cuando parezca que esté vacío.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Alvesco
- El principio activoes ciclesonida. Cada inhalación (dosis liberada por el inhalador) contiene 160 microgramos de ciclesonida.
- Los demás componentes (excipientes) sonetanol anhidro y un gas propulsor (HFA-134a, Norflurano).
- Este medicamento contiene gases fluorados de efecto invernadero.
Alvesco160 microgramos 30 inhalaciones - Cada inhalador contiene 4,31 g de HFA-134a, que corresponde a 0,006 toneladas de CO2 equivalente a (potencial de calentamiento global PCG = 1430).
Alvesco160 microgramos 60 inhalaciones - Cada inhalador contiene 5,59 g de HFA-134a, que corresponde a 0,008 toneladas de CO2 equivalente a (potencial de calentamiento global PCG = 1430).
Alvesco160 microgramos 120 inhalaciones - Cada inhalador contiene 8,80 g de HFA-134a, que corresponde a 0,013 toneladas de CO2 equivalente a (potencial de calentamiento global PCG = 1430).
Aspecto de Alvesco y contenido del envase
Alvesco consta de un líquido transparente e incoloro en un envase a presión de aluminio que libera a través de la boquilla una dosis precisa de ciclesonida en forma de aerosol.
Tamaños de los envases:
Inhalador con 30 inhalacionesmedidas con precisión
Inhalador con 60 inhalacionesmedidas con precisión
Inhalador con 120 inhalacionesmedidas con precisión
Cada inhalador contiene una dosis suficiente para 30, 60 o 120 inhalaciones. En función del número de inhalaciones diarias recomendadas por el médico:
- el inhalador con 30 inhalaciones contiene medicamento suficiente para cuatro semanas.
- el inhalador con 60 inhalaciones contiene medicamento suficiente para uno o dos meses.
- el inhalador con 120 inhalaciones contiene medicamento suficiente para dos o cuatro meses.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de fabricación:
Titular de la autorización de comercialización
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082 MA Ámsterdam
Países Bajos
Responsable de la fabricación:
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082 MA Ámsterdam
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Zentiva Spain S.L.U.
Paseo Club Deportivo 1, Edificio 4, Pozuelo de Alarcón 28223, Madrid
España
Este medicamento está autorizado en otros países de la UE con los siguientes nombres:
Austria | Alvesco 160, 80 Mikrogramm – Dosieraerosol |
Bulgaria | Alvesco 160, 80 micrograms, pressurised inhalation, solution |
Chipre | Alvesco 160, 80 μικρογραμμ?ρια, δι?λυμα για εισπνο? υπ? π?εση |
Croacia | Alvesco 160, 80 mikrograma stlaceni inhalat, otopina |
República Checa | Alvesco 160 mikrogramu/dávka roztok k inhalaci v tlakovém obalu |
Dinamarca | Alvesco 160, 80 mikrogram/dosis inhalationsspray, opløsning |
Finlandia | Alvesco 160, 80 mikrog/annos inhalaatiosumute, liuos |
Francia | Alvesco 160, 80 microgrammes/dose, solution pour inhalation en flacon pressurisé |
Alemania | Alvesco 160, 80 Mikrogramm Druckgasinhalation, Lösung |
Grecia | Alvesco 160, 80 μικρογραμμ?ρια, δι?λυμα για εισπνο? υπ? π?εση |
Hungría | Alvesco 160, 80 mikrogramm túlnyomásos inhalációs oldat |
Irlanda | Alvesco 160, 80 micrograms pressurised inhalation, solution |
Italia | Alvesco 160, 80 mcg soluzione pressurizzata per inalazione |
Letonia | Alvesco 160, 80 mikrogrami aerosols inhalacijam, zem spiediena, škidums |
Países Bajos | Alvesco 160, 80 Inhalator, aërosol, oplossing 160, 80 microgram/dosis |
Noruega | Alvesco 160, 80 mikrogram/dose inhalasjonsaerosol, oppløsning |
Polonia | Alvesco 160, 80 160, 80 μg/dawke inhalacyjna; aerozol inhalacyjny, roztwór |
Rumania | Alvesco 160, 80 Inhaler, 160, 80 micrograme/doza , solutie de inhalat presurizata |
Eslovaquia | Alvesco 160, 80 Inhalátor inhalacný roztok v tlakovom obale |
Eslovenia | Alvesco 160, 80 mikrogramov/odmerek inhalacijska raztopina pod tlakom |
España | Alvesco160 microgramos/inhalación solución para inhalación en envase a presión |
Suecia | Alvesco 160, 80 microgram/dos inhalationsspray, lösning |
Reino Unido (Irlanda del Norte) | Alvesco 160, 80 Inhaler |
Fecha de la última revisión de este prospecto:Octubre 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia49.17 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ALVESCO 160 microgramos/INHALACION SOLUCION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 200 mcgPrincipio activo: mometasoneFabricante: Organon Salud S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 400 µgPrincipio activo: mometasoneFabricante: Organon Salud S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 100 microgramos/pulsaciónPrincipio activo: beclometasoneFabricante: Laboratorio Aldo Union S.L.Requiere receta
Médicos online para ALVESCO 160 microgramos/INHALACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ALVESCO 160 microgramos/INHALACION SOLUCION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















