
ALOCARE 2,275 MG/ML SOLUCION PARA PULVERIZACION CUTANEA


Cómo usar ALOCARE 2,275 MG/ML SOLUCION PARA PULVERIZACION CUTANEA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Alocare 2,275 mg/ml solución para pulverización cutánea
finasterida
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Alocare y para qué se utiliza
- Qué necesita saber antes de empezar a usar Alocare
- Cómo usar Alocare
- Posibles efectos adversos
- Conservación de Alocare
- Contenido del envase e información adicional
1. Qué es Alocare y para qué se utiliza
Este medicamento contiene el principio activo finasterida. Se administra en la piel del cuero cabelludo calvo mediante un aplicador en spray que consiste en un frasco con una bomba y un cono.
Alocare se utiliza para el tratamiento de la pérdida de pelo masculino de leve a moderada (también conocida como alopecia androgenética). Este medicamento aumenta el crecimiento del pelo y evita que se siga perdiendo en los hombres. Alocare sólo puede ser utilizado en hombres de 18 a 41 años de edad.
La pérdida de pelo de tipo masculino es un trastorno frecuente que se cree que está causado por una combinación de factores genéticos y una hormona particular, llamada dihidrotestosterona (DHT).
Se cree que esta hormona contribuye a acortar la fase de crecimiento del pelo y hace que sea más fino.
A medida que los folículos pilosos se hacen más pequeños, la calvicie se hace evidente. Alocare reduce el nivel de DHT en el cuero cabelludo. Esto ayuda a invertir el proceso de calvicie, lo que conduce a un mayor crecimiento del pelo y a la prevención de una mayor pérdida del pelo.
2. Qué necesita saber antes de empezar a usar Alocare
No use Alocare
- Si es alérgico a finasterida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si es una mujer y esta o puede quedarse embarazada.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Alocare
Posible transmisión de Alocare
Si el principio activo se absorbe a través de la piel de una mujer que está embarazada de un bebé varón, este puede nacer con anormalidades en sus órganos sexuales.
Evite cualquier contacto entre la zona tratada y una mujer que esté o pueda quedar embarazada. También debe evitar tocar las superficies expuestas a este medicamento. Si se produce el contacto con Alocare, la mujer afectada debe lavarse la parte del cuerpo afectada de forma rápida y minuciosa.
Los niños y adolescentes no deben entrar en contacto con este medicamento. Si se produce el contacto con Alocare, el niño o el adolescente afectado debe lavarse la parte del cuerpo afectada de forma rápida y exhaustiva.
Efectos en el Antígeno Prostático Específico (APE)
Si le van a realizar un análisis de sangre del antígeno prostático específico (APE) para la detección del cáncer de próstata, informe a su médico de que está utilizando Alocare, ya que puede afectar a la interpretación de los resultados.
Efecto sobre la hormona masculina dihidrotestosterona (DHT)
Alocare disminuye la concentración de la hormona masculina (DHT) en sangre. Sin embargo, esto ocurre con poca frecuencia y también la disminución es menor que con los comprimidos de finasterida. Los efectos secundarios de naturaleza sexual que se conocen para los comprimidos de finasterida también pueden ocurrir con Alocare pero son menos probables (ver sección 4). Por lo tanto, respete la dosis que le ha recetado su médico. No utilice más de 4 pulverizaciones diarias.
Cáncer de mama
Aunque no se ha detectado cáncer de mama en los hombres tratados con Alocare en los estudios clínicos, se ha notificado durante el tratamiento con comprimidos de finasterida. Si experimenta cualquier cambio en el tejido de la mama, como bultos, dolor, aumento del tejido de la mama o secreción del pezón, póngase en contacto con su médico lo antes posible.
Alteraciones del estado de ánimo y depresión
Aunque no se han observado alteraciones del estado de ánimo en los pacientes tratados con Alocare en los estudios clínicos, si se han notificado durante el tratamiento con comprimidos de finasterida. Si experimenta síntomas como estado de ánimo deprimido, depresión o ideas de suicidio, póngase en contacto con su médico para que le aconseje lo antes posible.
Niños y adolescentes
Este medicamento no debe utilizarse en niños o adolescentes. No hay datos que demuestren la eficacia y la seguridad de la finasterida en los niños menores de 18 años.
Otros medicamentos y Alocare
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No aplique Alocare si está utilizando otros productos tópicos, como cosméticos, protectores solares u otros medicamentos, en la misma zona.
Embarazo
Las mujeres no deben tomar Alocare.
Las mujeres que estén o puedan estar embarazadas deben evitar el contacto con el cuero cabelludo tratado o con las superficies expuestas a Alocare. Véase la sección "Posible trasnmision de Alocare" más arriba. Si se produce un contacto directo con este medicamento, la mujer debe lavarse la parte del cuerpo afectada rápida y minuciosamente y pedir consejo a su médico.
Conducción y uso de máquinas
Alocare no influye en la capacidad para conducir o manejar maquinaria.
Alocare contiene Etanol
Este medicamento contiene 25 mg de etanol (96%) por cada pulverización, lo que equivale a 0,5 mg/microlitro (55%). Puede causar sensacion de ardor en piel lesionada.
3. Cómo usar Alocare
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dependiendo de la extensión de su cuero cabelludo sin pelo, su médico le recetará entre 1 y 4 pulverizaciones diarias que deberá utilizar en zonas distintas. No utilice más de 4 pulverizaciones diarias.
Este medicamento es de uso cutáneo. Sólo debe utilizarse en el cuero cabelludo.
Alocare se compone de dos componentes separados: un frasco con una bomba acoplada y un cono. Estos componentes requieren ser ensamblados antes de su primer uso. Antes de la primera aplicación, lea todas las instrucciones que se indican a continuación.
Asegúrese de que el cabello y el cuero cabelludo estén completamente secos antes de la aplicación. Aplique Alocare en el cuero cabelludo por si solo. . Si se prescribe más de una pulverización, aplíquelas en zonas que no se superpongan. No aplique la solución en otras zonas del cuerpo que no sean el cuero cabelludo. Una vez aplicado, deje actuar Alocare durante al menos 6 horas.
Este medicamento puede transferirse por contacto con tejidos, manos u otras superficies y objetos. Evite el contacto del cuero cabelludo tratado con almohadas, cascos, sombreros, etc. hasta que la solución se haya secado.
Alocare puede transferirse desde su propio cuerpo a otros si tocan su cuero cabelludo tratado u otras superficies expuestas. Si se produce el contacto con Alocare, la persona debe lavarse la parte del cuerpo afectada de forma rápida y exhaustiva.
Guarde Alocare en un lugar seguro fuera del alcance de los niños. Advierta a los miembros de la familia o a otras personas con acceso al lugar de almacenamiento de las precauciones de contacto.
Componentes y montaje del pulverizador
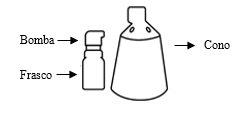
Montaje del pulverizador
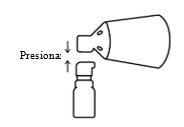 Alinear y presionar
Alinear y presionar
Alinee el cono con el botón de la bomba y presione firmemente.
- Montaje correcto
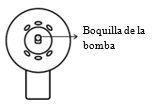 El aplicador de pulverización está correctamente montado cuando se oye un clic después de presionar, la boquilla de pulverización de la bomba se encuentra en la posición central del cono
El aplicador de pulverización está correctamente montado cuando se oye un clic después de presionar, la boquilla de pulverización de la bomba se encuentra en la posición central del cono
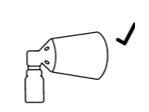 Montaje correcto
Montaje correcto
y la parte inferior del botón de la bomba está alineada con la parte inferior del cono sin separación.
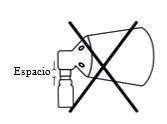 Montaje incorrecto si hay un hueco. Vuelva a alinear y presione de nuevo
Montaje incorrecto si hay un hueco. Vuelva a alinear y presione de nuevo
Si no se oye un clic durante el montaje o se ve un hueco entre la parte inferior del botón de la bomba y el cono, vuelva a alinear los componentes y presione en su lugar de nuevo.
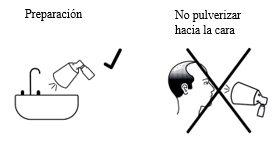
Preparación de la bomba
- Tras el montaje del aplicador de pulverización, se tiene que preparar la bomba para el primer uso. Si el aplicador de pulverización no se ha utilizado durante 2 semanas o más, será necesario volver a preparar la bomba. No es necesario volver a prepararla en cada uso.
- Para preparar la bomba por primera vez, presione a fondo la bomba cuatro veces con el pulgar o el dedo índice, dirigiendo la solución pulverizada a un fregadero. A continuación, enjuague el fregadero con agua. Para volver a preparar la bomba después de no usarla durante 2 semanas o más, presione completamente la bomba una sola vez.
- No rocíe Alocare hacia la cara.
Si se desprende solución durante el montaje o el preparado, limpie las superficies en las que pueda haberse depositado
Aplicación de la dosis
- Dependiendo del tamaño de la zona sin pelo del cuero cabelludo, su médico le recetará entre 1 y 4 pulverizaciones diarias.
- No es necesario agitar el frasco antes de utilizarlo.
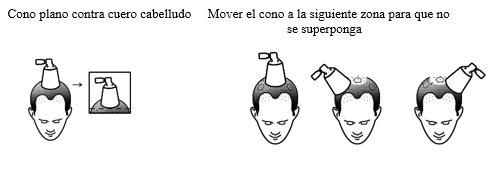
- Sujete el aplicador del spray con el cono plano contra el cuero cabelludo para evitar la dispersión de la solución en el aire.
- Presione completamente la bomba una vez para una pulverización.
- Mueva el cono a diferentes zonas del cuero cabelludo para aplicar otras dosis según el número de pulverizaciones prescritas por su médico. No superponga y trate las zonas que ya han sido rociadas.
- Después del uso, no retire el cono de la bomba. Vuelva a colocar el aplicador de pulverización en la caja.
- Una vez aplicado, no enjuague Alocare durante al menos 6 horas.
Asegúrese de que Alocare no entre en contacto con sus manos ni con ninguna otra parte del cuerpo. Lave inmediatamente y a fondo cualquier parte del cuerpo expuesta que no sea su cuero cabelludo.
Si el cono se ensucia, límpielo con un pañuelo de papel limpio y seco. Tire de forma segurael pañuelo usado y lávese bien las manos.
Dosis y días de tratamiento por dosis
El frasco contiene hasta 180 pulverizaciones. El número de días de tratamiento depende de la dosis prescrita, de 1 a 4 pulverizaciones al día. No utilice el frasco más allá de las 180 pulverizaciones, ya que la solución restante en el frasco podría no dar una dosis completa, lo que podría limitar el efecto de su tratamiento.
Pulverizaciones por día | Días de tratamiento |
1 | 180 |
2 | 90 |
3 | 60 |
4 | 45 |
- El farmacéutico anotará en la caja la dosis prescrita y el número restante de días de tratamiento hasta que se agote el producto.
- En la fecha de inicio del tratamiento con Alocare, anote en su calendario la dosis prescrita (de 1 a 4 pulverizaciones) y calcule cuándo necesitará un nuevo frasco. Póngase en contacto con su médico antes de que se agote su suministro para que no haya una interrupción del tratamiento.
Si usa más Alocare del que debe
Si se aplica más Alocare del recomendado, habla con tu médico. Alocare no funcionará más rápido o mejor si se aplica más de una vez al día, pero los efectos secundarios se pueden producir con mayor probabilidad.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada.
Si olvidó tomar Alocare
Si se olvida de aplicar Alocare, no aplique una dosis doble para compensar la aplicación olvidada. Siga utilizando la dosis recomendada por su médico.
Si interrumpe el tratamiento con Alocare
El efecto del tratamiento puede tardar 3 meses en desarrollarse. Es importante que siga utilizando Alocare durante el tiempo que le indique su médico. Si deja de aplicarse Alocare, es probable que pierda el pelo que ha ganado.
Si tiene alguna otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Picor o enrojecimiento del cuero cabelludo
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Disminución de una hormona masculina (dihidrotestosterona) en sangre
Otros efectos secundarios conocidos con la finasterida oral también se pueden producir con Alocare.
Esto incluye:
- Reacciones alérgicas (de hipersensibilidad) que incluyen erupción cutánea, picor, hinchazón alrededor de la boca (angioedema)
- Estado de ánimo deprimido
- Ansiedad
- Sensación de latidos del corazón (palpitaciones)
- Aumento de las enzimas hepáticas
- Sensibilidad y aumento de las mamas
- Dolor en los testículos
- Sangre en la eyaculación (hematospermia)
- Deterioro del deseo sexual
- Dificultad para tener una erección
- Trastorno de la eyaculación, incluida la disminución de la cantidad de eyaculación
- Infertilidad
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico incluso si se tratan de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es . Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Alocare
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche, después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Alocare contiene alcohol y, por tanto, es inflamable. Evite rociar el medicamento cerca de llamas abiertas o mientras fuma.
No utilice Alocare durante más de 6 meses después de abrir el frasco por primera vez.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Alocare
- El principio activo es finasterida. Cada mL de solución contiene 2,275 mg de finasterida. Cada pulverización proporciona 50 microlitros, que contienen 114 microgramos de finasterida.
- Los demás componentes son: etanol (96%), agua purificada, propilenglicol, hidroxipropil quitosano.
Aspecto del producto y contenido del envase
Alocare es una solución cutánea en spray incolora, transparente y ligeramente viscosa.
Tamaño del envase:
- 1 frasco (correspondiente a 180 pulverizaciones) con una bomba mecánica de pulverización a presión y 1 cono separado.
- 3 frascos (correspondientes a 3 x 180 pulverizaciones) con una bomba mecánica de pulverización a presión y 3 conos separados.
Antes de la primera utilización, fije el cono a la bomba del frasco, como se describe en el apartado 3.
Puede que solamente estén comercializado algunos tamaños de envase
Titular de la autorización de comercialización
Industrial Farmacéutica Cantabria, S.A.
Ctra. Cazoña-Adarzo, s/n
39011 Santander
España
Responsable de la fabricación
Almirall Hermal GmbH
Scholtzstrasse 1 and 3- Reinbek
Schleswig-Holstein – 21465
Alemania
Este medicamento está autorizado en los Estados miembros del EEE con las siguientes denominaciones:
Country | Trade names |
Germany | Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung |
Bulgaria | ?????? ?? ???? 2,275 mg/ml ????? ?? ????, ??????? Finjuve for men 2.275 mg/ml cutaneous spray, solution |
Czech Republic | Fynzur 2.275 mg/ml kožní sprej, roztok |
Hungary | Fynzur férfiaknak 2,275 mg/ml külsoleges oldatos spray |
Italy | CARETOPIC 2.275 mg/ml spray cutaneo, soluzione |
Luxembourg | Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung |
Poland | Finjuve, 2,275 mg/ml, aerozol na skóre, roztwór |
Portugal | Finasterida Cantabria 2.275 mg/ml Solução para Pulverização Cutânea |
Romania | Finjuve pentru barba?i 2,275 mg/ml spray cutanat, solutie |
Slovakia | Finjuve pre mužov 2,275 mg/ml dermálny roztokový sprej |
Spain | Alocare 2,275 mg/ml Solución para pulverización cutánea |
Fecha de la última revisión de este prospecto:Febrero 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ALOCARE 2,275 MG/ML SOLUCION PARA PULVERIZACION CUTANEAForma farmacéutica: COMPRIMIDO, 1 mgPrincipio activo: FinasteridaFabricante: Industrial Farmaceutica Cantabria S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 1 mg / comprimidoPrincipio activo: FinasteridaFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 1 mgPrincipio activo: FinasteridaFabricante: Laboratoires Bailleul S.A.Requiere receta
Médicos online para ALOCARE 2,275 MG/ML SOLUCION PARA PULVERIZACION CUTANEA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ALOCARE 2,275 MG/ML SOLUCION PARA PULVERIZACION CUTANEA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








