
AIDRANA 3 MG/0,02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG


Cómo usar AIDRANA 3 MG/0,02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la usuaria
aidrana 3 mg/ 0,02 mg comprimidos recubiertos con película EFG
drospirenona/etinilestradiol
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
Cosas importantes que debe saber acerca de los anticonceptivos hormonales combinados (AHCs):
- Son uno de los métodos anticonceptivos reversibles más fiables si se utilizan correctamente.
- Aumentan ligeramente el riesgo de sufrir un coágulo de sangre en las venas y arterias, especialmente en el primer año o cuando se reinicia el uso de un anticonceptivo hormonal combinado tras una pausa de 4 semanas o más.
- Esté alerta y consulte a su médico si cree que puede tener síntomas de un coágulo de sangre (ver sección 2 “Coágulos de sangre”).
Contenido del prospecto
- Qué es aidrana y para qué se utiliza
- Qué necesita saber antes de empezar a tomar aidrana
- Cómo tomar aidrana
- Posibles efectos adversos
- Conservación de aidrana
- Contenido del envase e información adicional
1. Qué es aidrana y para qué se utiliza
- aidrana es un anticonceptivo que se utiliza para evitar el embarazo.
- Cada uno de los 24 comprimidos rosa contiene una pequeña cantidad de dos hormonas femeninas diferentes, denominadas drospirenona y etinilestradiol.
- Los 4 comprimidos blancos no contienen principios activos y también se denominan comprimidos de placebo.
Los anticonceptivos que contienen dos hormonas se denominan anticonceptivos combinados.
2. Qué necesita saber antes de empezar a tomar aidrana
Consideraciones generales Antes de empezar a usar aidrana debe leer la información acerca de los coágulos de sangre en la sección 2. Es particularmente importante que lea los síntomas de un coágulo de sangre (ver sección 2 “Coágulos de sangre”). Antes de empezar a tomar aidrana, su médico le hará algunas preguntas sobre su historia clínica personal y familiar. El médico también medirá su presión arterial, y dependiendo de su situación personal, podrá llevar a cabo algunas otras pruebas. En este prospecto se describen varias situaciones en las que usted debería de interrumpir el uso de aidrana, o en las que la fiabilidad de aidrana puede disminuir. En dichas situaciones, usted no debería tener relaciones sexuales o debería tomar precauciones anticonceptivas adicionales no hormonales, por ejemplo, uso de preservativo u otro método de barrera. No utilice el método del ritmo o el de la temperatura. Estos métodos pueden no ser fiables puesto que drospirenona/etinilestradiol altera los cambios mensuales de la temperatura corporal y del moco cervical. aidrana,al igual que otros anticonceptivos hormonales, no protege frente a la infección por VIH (SIDA) o cualquier otra enfermedad de transmisión sexual. |
Cuándo no debe usaraidrana
No debe usar aidrana si tiene alguna de las afecciones enumeradas a continuación. Informe a su médico si tiene alguna de las afecciones enumeradas a continuación. Su médico comentará con usted qué otra forma de anticoncepción sería más adecuada.
No tome aidrana:
- Si tiene (o ha tenido alguna vez) un coágulo de sangre en un vaso sanguíneo de las piernas (trombosis venosa profunda, TVP), en los pulmones (embolia pulmonar, EP) o en otros órganos.
- Si sabe que padece un trastorno que afecta a la coagulación de la sangre: por ejemplo, deficiencia de proteína C, deficiencia de proteína S, deficiencia de antitrombina III, factor V Leiden o anticuerpos antifosfolípidos.
- Si necesita una operación o si pasa mucho tiempo sin ponerse de pie (ver sección “Coágulos de sangre”).
- Si ha sufrido alguna vez un ataque al corazón o un ictus.
- Si tiene (o ha tenido alguna vez) una angina de pecho (una afección que provoca fuerte dolor en el pecho y puede ser el primer signo de un ataque al corazón) o un accidente isquémico transitorio (AIT, síntomas temporales de ictus).
- Si tiene alguna de las siguientes enfermedades que pueden aumentar su riesgo de formación de un coágulo en las arterias:
- Diabetes grave con lesión de los vasos sanguíneos.
- Tensión arterial muy alta.
- Niveles muy altos de grasa en la sangre (colesterol o triglicéridos).
- Una afección llamada hiperhomocisteinemia.
- Si tiene (o ha tenido alguna vez) un tipo de migraña llamada “migraña con aura”.
- Si tiene (o ha tenido en el pasado) una enfermedad del hígado y su función hepática no se ha normalizado todavía.
- Si sus riñones no funcionan bien (insuficiencia renal).
- Si tiene (o ha tenido en el pasado) un tumor en el hígado.
- Si tiene (o ha tenido en el pasado), o se sospecha que tiene cáncer de mama o cáncer de los órganos sexuales.
- Si tiene hemorragias vaginales, cuya causa es desconocida.
- Si es alérgica a drospirenona o etinilestradiol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Esto puede manifestarse con picor, erupción o inflamación.
- Si tiene hepatitis C y está tomando medicamentos que contienen ombitasvir/paritaprevir/ritonavir y dasabuvir, glecaprevir / pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir (ver también la sección “Toma deaidranacon otros medicamentos”).
Advertencias y precauciones
¿Cuándo debe consultar a su médico? Busque asistencia médica urgente
Para obtener una descripción de los síntomas de estos efectos adversos graves, consulte “Cómo reconocer un coágulo de sangre”. |
Informe a su médico si sufre cualquiera de las siguientes afecciones.
En algunas situaciones, usted deberá tener especial cuidado mientras use drospirenona/etinilestradiol o cualquier otro anticonceptivo hormonal combinado, y puede ser necesario que su médico le examine de forma periódica. Si la afección se desarrolla o empeora mientras está usando drospirenona/etinilestradiol, también debe informar a su médico
- Si algún familiar cercano tiene o ha tenido alguna vez cáncer de mama.
- Si tiene alguna enfermedad del hígado o de la vesícula biliar.
- Si tiene diabetes
- Si tiene depresión.
- Si tiene enfermedad de Crohn o colitis ulcerosa (enfermedad intestinal inflamatoria crónica).
- Si tiene lupus eritematoso sistémico (LES, una enfermedad que afecta a su sistema natural de defensa).
- Si tiene síndrome urémico hemolítico (SUH, un trastorno de la coagulación de la sangre que provoca insuficiencia en el riñón).
- Si tiene anemia de células falciformes (una enfermedad hereditaria de los glóbulos rojos).
- Si tiene niveles elevados de grasa en la sangre (hipertrigliceridemia) o antecedentes familiares conocidos de esta afección. La hipertrigliceridemia se ha asociado a un mayor riesgo de padecer pancreatitis (inflamación del páncreas).
- Si necesita una operación o pasa mucho tiempo sin ponerse de pie (ver sección 2 “Coágulos de sangre”).
- Si acaba de dar a luz corre mayor riesgo de sufrir coágulos de sangre. Debe preguntar a su médico cuándo puede empezar a tomar aidrana tras el parto.
- Si tiene una inflamación de las venas que hay debajo de la piel (tromboflebitis superficial). Si tiene varices.
- Si tiene epilepsia (ver “Toma de aidrana con otros medicamentos”).
- Si tiene algunas enfermedades que aparecieron por primera vez durante el embarazo o en un anterior uso de hormonas sexuales (por ejemplo, pérdida de audición, una enfermedad de la sangre llamada porfiria, erupción cutánea con vesículas durante el embarazo (herpes gestacional), una enfermedad nerviosa en la que aparecen movimientos involuntarios (corea de Sydenham)). Si tiene o ha tenido alguna vez manchas de color pardo dorado (cloasma), también llamadas “manchas del embarazo”, especialmente en la cara. En este caso, evite la exposición directa al sol o a los rayos ultravioleta.
- Si experimenta síntomas de angioedema como hinchazón de la cara, lengua y/o garganta, y/o dificultad para tragar o urticaria, con posible dificultad para respirar, contacte con un médico de forma inmediata. Los productos que contienen estrógenos pueden causar o empeorar los síntomas del angioedema hereditario y adquirido.
Consulte a su médico o farmacéutico antes de empezar a tomar aidrana.
Trastornos psiquiátricos:
Algunas mujeres que utilizan anticonceptivos hormonales como aidrana han notificado depresión o un estado de ánimo deprimido. La depresión puede ser grave y a veces puede inducir pensamientos suicidas. Si experimenta alteraciones del estado de ánimo y síntomas depresivos, póngase en contacto con su médico para obtener asesoramiento médico adicional lo antes posible.
COÁGULOS DE SANGRE
El uso de un anticonceptivo hormonal combinado como aidrana aumenta su riesgo de sufrir un coágulo de sangreen comparación con no usarlo. En raras ocasiones un coágulo de sangre puede bloquear vasos sanguíneos y provocar problemas graves.
Se pueden formar coágulos de sangre:
- En las venas (lo que se llama “trombosis venosa”, “tromboembolismo venoso” o TEV).
- En las arterias (lo que se llama “trombosis arterial”, “tromboembolismo arterial” o TEA).
La recuperación de los coágulos de sangre no es siempre completa. En raras ocasiones puede haber efectos graves duraderos o, muy raramente, pueden ser mortales.
Es importante recordar que el riesgo global de un coágulo de sangre perjudicial debido a aidrana es pequeño.
CÓMO RECONOCER UN COÁGULO DE SANGRE
Busque asistencia médica urgente si nota alguno de los siguientes signos o síntomas.
¿Experimenta alguno de estos signos? | ¿Qué es posible que esté sufriendo? |
Cambio de color de la piel de la pierna, p. ej. si se pone pálida, roja o azul. | Trombosis venosa profunda |
Si no está segura, consulte a un médico, ya que algunos de estos síntomas como la tos o la falta de aliento se pueden confundir con una afección más leve como una infección respiratoria (p. ej. un “catarro común”). | Embolia pulmonar |
Síntomas que se producen con más frecuencia en un ojo:
| Trombosis de las venas retinianas (coágulo de sangre en el ojo). |
| Ataque al corazón. |
A veces los síntomas de un ictus pueden ser breves, con una recuperación casi inmediata y completa, pero de todos modos debe buscar asistencia médica urgente ya que puede correr riesgo de sufrir otro ictus. | Ictus |
| Coágulos de sangre que bloquean otros vasos sanguíneos. |
COÁGULOS DE SANGRE EN UNA VENA
¿Qué puede ocurrir si se forma un coágulo de sangre en una vena?
- El uso de anticonceptivos hormonales combinados se ha relacionado con un aumento del riesgo de coágulos de sangre en las venas (trombosis venosa). No obstante, estos efectos adversos son raros. Se producen con más frecuencia en el primer año de uso de un anticonceptivo hormonal combinado.
- Si se forma un coágulo de sangre en una vena de la pierna o del pie, puede provocar trombosis venosa profunda (TVP).
- Si un coágulo de sangre se desplaza desde la pierna y se aloja en el pulmón puede provocar una embolia pulmonar.
- En muy raras ocasiones se puede formar un coágulo en una vena de otro órgano como el ojo (trombosis de las venas retinianas)
¿Cuándo es mayor el riesgo de presentar un coágulo de sangre en una vena?
El riesgo de presentar un coágulo de sangre en una vena es mayor durante el primer año en el que se toma un anticonceptivo hormonal combinado por primera vez. El riesgo puede ser mayor también si vuelve a empezar a tomar un anticonceptivo hormonal combinado (el mismo medicamento o un medicamento diferente) después de una interrupción de 4 semanas o más.
Después del primer año, el riesgo disminuye, pero siempre es algo mayor que si no estuviera tomando un anticonceptivo hormonal combinado.
Cuando deja de tomar aidrana, su riesgo de presentar un coágulo de sangre regresa a la normalidad en pocas semanas.
¿Cuál es el riesgo de presentar un coágulo de sangre?
El riesgo depende de su riesgo natural de TEV y del tipo de anticonceptivo hormonal combinado que esté tomando.
El riesgo global de presentar un coágulo de sangre en la pierna o en el pulmón (TVP o EP) con aidrana es pequeño.
- De cada 10.000 mujeres que no usan un anticonceptivo hormonal combinado y que no están embarazadas, unas 2 presentarán un coágulo de sangre en un año.
- De cada 10.000 mujeres que usan un anticonceptivo hormonal combinado que contiene levonorgestrel, noretisterona o norgestimato, unas 5-7 presentarán un coágulo de sangre en un año.
- De cada 10.000 mujeres que usan un anticonceptivo hormonal combinado que contiene drospirenona como drospirenona/etinilestradiol, entre unas 9 y 12 mujeres presentarán un coágulo de sangre en un año.
- El riesgo de presentar un coágulo de sangre dependerá de sus antecedentes personales (ver “Factores que aumentan su riesgo de un coágulo sanguíneo” más adelante).
Riesgo de presentar un coágulo de sangre en un año | |
Mujeres que no utilizanun comprimido hormonal combinado y que no están embarazadas | Unas 2 de cada 10.000 mujeres |
Mujeres que utilizan un comprimido anticonceptivo hormonal combinado que contiene levonorgestrel, noretisterona o norgestimato | Unas 5-7 de cada 10.000 mujeres |
Mujeres que utilizan aidrana | Unas 9-12 de cada 10.000 mujeres |
Factores que aumentan su riesgo de un coágulo de sangre en una vena
El riesgo de tener un coágulo de sangre con aidrana es pequeño, pero algunas afecciones aumentan el riesgo. Su riesgo es mayor:
- Si tiene exceso de peso (índice de masa corporal o IMC superior a 30 kg/m2).
- Si alguno de sus parientes próximos ha tenido un coágulo de sangre en la pierna, pulmón u otro órgano a una edad temprana (es decir, antes de los 50 años aproximadamente). En este caso podría tener un trastorno hereditario de la coagulación de la sangre.
- Si necesita operarse o si pasa mucho tiempo sin ponerse de pie debido a una lesión o enfermedad o si tiene la pierna escayolada. Tal vez haya que interrumpir el uso de aidrana varias semanas antes de la intervención quirúrgica o mientras tenga menos movilidad. Si necesita interrumpir el uso de aidrana pregúntele a su médico cuándo puede empezar a usarlo de nuevo.
- Al aumentar la edad (en especial por encima de unos 35 años).
- Si ha dado a luz hace menos de unas semanas.
El riesgo de presentar un coágulo de sangre aumenta cuantas más afecciones tenga.
Los viajes en avión (más de 4 horas) pueden aumentar temporalmente el riesgo de un coágulo de sangre, en especial si tiene alguno de los demás factores de riesgo enumerados.
Es importante informar a su médico si sufre cualquiera de las afecciones anteriores, aunque no esté segura. Su médico puede decidir que hay que interrumpir el uso de aidrana.
Si alguna de las afecciones anteriores cambia mientras está utilizando aidrana, por ejemplo un pariente próximo experimenta una trombosis sin causa conocida o usted aumenta mucho de peso, informe a su médico
COÁGULOS DE SANGRE EN UNA ARTERIA
¿Qué puede ocurrir si se forma un coágulo de sangre en una arteria?
Al igual que un coágulo de sangre en una vena, un coágulo en una arteria puede provocar problemas graves. Por ejemplo, puede provocar un ataque al corazón o un ictus.
Factores que aumentan su riesgo de un coágulo de sangre en una arteria
Es importante señalar que el riesgo de un ataque al corazón o un ictus por utilizar drospirenona/etinilestradiol es muy pequeño, pero puede aumentar:
- Con la edad (por encima de unos 35 años).
- Si fuma.Cuando utiliza un anticonceptivo hormonal combinado como aidrana se le aconseja que deje de fumar. Si no es capaz de dejar de fumar y tiene más de 35 años, su médico puede aconsejarle que utilice un tipo de anticonceptivo diferente.
- Si tiene sobrepeso.
- Si tiene la tensión arterial alta.
- Si algún pariente próximo ha sufrido un ataque al corazón o un ictus a una edad temprana (menos de unos 50 años). En este caso usted también podría tener mayor riesgo de sufrir un ataque al corazón o un ictus.
- Si usted o alguno de sus parientes próximos tiene un nivel elevado de grasa en la sangre (colesterol o triglicéridos).
- Si padece migrañas, especialmente migrañas con aura.
- Si tiene un problema de corazón (trastorno de las válvulas, alteración del ritmo cardíaco llamado fibrilación auricular).
- Si tiene diabetes.
Si tiene más de una de estas afecciones o si alguna de ellas es especialmente grave, el riesgo de presentar un coágulo de sangre puede verse incrementado aún más.
Si alguna de las afecciones anteriores cambia mientras está utilizando aidrana, por ejemplo empieza a fumar, un pariente próximo experimenta una trombosis sin causa conocida o usted aumenta mucho de peso, informe a su médico.
Drospirenona/etinilestradiol ycáncer
Se ha observado cáncer de mama ligeramente más a menudo en mujeres que usan anticonceptivos combinados, pero no se sabe si esto se debe al tratamiento. Por ejemplo, puede ser que se detecten más tumores en mujeres que usan anticonceptivos combinados porque son examinadas por el médico más a menudo. La incidencia de tumores de mama disminuye gradualmente después de dejar de tomar anticonceptivos hormonales combinados. Es importante someterse regularmente a exámenes de las mamas y usted debe acudir a su médico si nota cualquier bulto.
En raras ocasiones se han comunicado tumores benignos en el hígado, y más raramente tumores malignos, en usuarias de anticonceptivos. Acuda a su médico si usted sufre un dolor abdominal inusualmente fuerte.
Sangrado entre periodos
Durante los primeros meses en los que usted está tomando aidrana, puede tener sangrados inesperados (sangrados fuera de los días de uso de comprimidos placebo). Si estos sangrados persisten más allá de unos meses, o comienzan tras unos meses, su médico debe investigar la causa.
Qué debe hacer si no tiene la regla durante los días de placebo
Si usted ha tomado correctamente todos los comprimidos activos de color rosa, no ha tenido vómitos o diarrea intensa y tampoco ha tomado otros medicamentos, es muy improbable que esté embarazada.
Si la regla prevista no le viene en dos ocasiones consecutivas, usted puede estar embarazada. Acuda a su médico inmediatamente. No comience con el siguiente blíster hasta que no esté segura de que no está embarazada.
Toma de aidrana con otros medicamentos
Informe al médico que le haya prescrito aidrana en todo momento sobre medicamentos o preparados a base de hierbas que está tomando. También informe a cualquier otro médico o dentista que le recete otro medicamento (o a su farmacéutico) de que toma aidrana. Ellos pueden indicarle si usted necesita tomar precauciones anticonceptivas adicionales (por ejemplo, preservativos) y, si es así, durante cuánto tiempo, o si debe modificar el uso de otro medicamento que necesite. |
Algunos medicamentos
- pueden tener una influencia en los niveles de aidrana en sangre
- pueden hacer que sea menos efectivo en la prevención del embarazo
- pueden causar sangrados inesperados
Esto puede ocurrir con
- medicamentos utilizados en el tratamiento de:
- la epilepsia (p. ej. primidona, fenitoína, barbitúricos, carbamazepina, oxcarbazepina)
- la tuberculosis (p. ej. rifampicina)
- las infecciones por el VIH y el virus de la Hepatitis C (los llamados inhibidores de la proteasa e inhibidores no nucleósidos de la transcriptasa inversa, tales como ritonavir, nevirapina, efavirenz)
- infecciones fúngicas (p. ej. griseofulvina, ketoconazol)
- artritis, artrosis (etoricoxib)
- la presión alta en los vasos sanguíneos de los pulmones (bosentán)
- los preparados a base de hierba de San Juan
Drospirenona/etinilestradiol puede influir sobre el efectode otros medicamentos, por ejemplo:
- medicamentos que contienen ciclosporina
- el antiepiléptico lamotrigina (puede llevar a un aumento de la frecuencia de convulsiones)
- teofilina (usada para tratar problemas respiratorios)
- tizanidina (usada para tratar dolores y/o calambres musculares)
Notome aidranasi usted tiene hepatitis C y está tomando medicamentos que contienen ombitasvir/paritaprevir/ritonavir y dasabuvir, glecaprevir / pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir, ya que estos medicamentos pueden producir aumentos en los resultados de pruebas hepáticas (aumento de la enzima hepática ALT).
Su médico le prescribirá otro tipo de anticonceptivo antes de comenzar el tratamiento con estos medicamentos.
aidrana se puede volver a usar aproximadamente 2 semanas después de la finalización de este tratamiento. Consulte la sección "No tome aidrana").
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento
Toma de aidrana con alimentos y bebidas
Drospirenona/etinilestradiol se puede tomar con o sin alimentos, y con algo de agua si fuera necesario.
Pruebas de laboratorio
Si usted necesita un análisis de sangre, comente con su médico o con el personal del laboratorio que está tomando un anticonceptivo, ya que los anticonceptivos orales pueden influir en los resultados de algunas pruebas.
Embarazo y lactancia
Embarazo
Si usted está embarazada, no debe tomar aidrana. Si se queda embarazada durante el tratamiento con aidrana debe interrumpir el tratamiento inmediatamente y ponerse en contacto con su médico. Si usted desea quedarse embarazada, puede dejar de tomar aidrana en cualquier momento (ver “Si interrumpe el tratamiento con aidrana”).
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Lactancia
En general, no se recomienda tomar aidrana durante el periodo de lactancia. Si usted quiere tomar el anticonceptivo mientras está en periodo de lactancia, debe consultar con su médico.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
No hay información que sugiera que el uso de aidrana tenga algún efecto sobre la capacidad para conducir o para utilizar máquinas.
aidrana contiene lactosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar aidrana
Cada blíster contiene 24 comprimidos activos de color rosa y 4 comprimidos blancos de placebo. Los comprimidos de aidrana de distintos colores están ordenados. Un blíster contiene 28 comprimidos.
Tome un comprimido de aidrana todos los días, con algo de agua si fuera necesario. Puede tomar los comprimidos con o sin comida, pero todos los días aproximadamente a la misma hora.
No confunda los comprimidos: tome un comprimido de color rosa durante los primeros 24 días y después un comprimido blanco durante los últimos 4 días. Después debe comenzar otro blíster inmediatamente (24 comprimidos de color rosa y después 4 comprimidos blancos). Por lo tanto, no hay intervalo entre dos blísteres.
Dado que la composición de los comprimidos es distinta, tiene que comenzar el blíster por el comprimido de la esquina superior izquierda y tomar los comprimidos todos los días. Siga la dirección de las flechas en el blíster para tomar los comprimidos en el orden correcto.
Preparación del blíster
Para ayudarle a seguir el orden de toma, cada envase de aidrana contiene 7 tiras autoadhesivas con los 7 días de la semana. Escoja la tira de la semana que empieza con el día en que toma el primer comprimido. Por ejemplo, si toma su primer comprimido un miércoles, utilice la tira que empieza con “MIE”.
Pegue la tira adhesiva de la semana en la parte superior del blíster de aidrana, donde se indica “¡Coloque la tira adhesiva aquí!”, de forma que el primer día esté colocado por encima del comprimido marcado con “1”.
Ahora tendrá un día marcado por encima de cada comprimido y podrá comprobar visualmente si ha tomado su comprimido. Las flechas indican el orden de toma de los comprimidos.
A lo largo de los 4 días en que se toman los comprimidos de placebo (los días placebo), debería tener lugar la menstruación (la denominada hemorragia por privación). Habitualmente comienza el 2º o 3er día después de tomar el último comprimido activo de color rosa de aidrana. Después de haber tomado el último comprimido blanco, comience el siguiente blíster, aunque la menstruación no haya finalizado. Esto quiere decir que usted debe comenzar el siguiente blíster en el mismo día de la semana en que inició el anterior y que la menstruación por privación debe tener lugar durante los mismos días cada mes.
Si usted usa aidrana de este modo, también está protegida frente al embarazo durante los 4 días en los que toma el comprimido placebo.
Cuándo puede empezar con el primer blíster
- Si usted no ha usado ningún anticonceptivo hormonal en el mes anterior
Comience a tomar aidrana el primer día del ciclo (es decir, el primer día de su menstruación). Si comienza aidrana el primer día de su menstruación, está protegida inmediatamente frente a un embarazo. También puede comenzar los días 2–5 del ciclo, pero en ese caso debe utilizar métodos anticonceptivos adicionales (por ejemplo, un preservativo) durante los 7 primeros días.
- Cambio desde un anticonceptivo hormonal combinado, anillo anticonceptivo combinado vaginal o parche
Usted puede comenzar a tomar aidrana preferentemente el día después de tomar el último comprimido activo (el último comprimido que contiene principios activos) de su anticonceptivo anterior, pero a más tardar al día siguiente de los días sin comprimidos (o después del último comprimido inactivo) de su anticonceptivo anterior. Cuando cambie desde un anillo anticonceptivo combinado vaginal o parche, siga las recomendaciones de su médico.
- Cambio desde un método basado exclusivamente en progestágenos (píldora de progestágenos solos, inyección, implante o sistema de liberación intrauterino (SLI) de progestágenos)
Puede cambiar desde la píldora de progestágenos solos cualquier día. Si se trata de un implante o un SLI, el mismo día de su retirada; si se trata de un inyectable, cuando corresponda la siguiente inyección. En todos los casos es recomendable que utilice medidas anticonceptivas adicionales (por ejemplo, un preservativo) durante los 7 primeros días de toma de comprimidos.
- Después de un aborto
Siga las recomendaciones de su médico.
- Después de tener un niño
Tras tener un niño, puede comenzar a tomar aidrana entre 21 y 28 días después. Si usted comienza más tarde, debe utilizar uno de los métodos denominados de barrera (por ejemplo, un preservativo) durante los 7 primeros días del uso de aidrana.
Si, después de tener un niño, usted ya ha tenido relaciones sexuales, antes de comenzar a tomar aidrana, usted debe estar segura de no estar embarazada o esperar a su siguiente periodo menstrual.
- Si usted está en periodo de lactancia y quiere empezar a tomar drospirenona/etinilestradiol
Lea la sección “Lactancia”.
Consulte a su médico si tiene dudas sobre cuando empezar.
Si toma másaidranadel que debe
No se han comunicado casos en los que la ingestión de una sobredosis de aidrana haya causado daños graves.
Si usted toma muchos comprimidos a la vez, puede encontrarse mal o tener vómitos o hemorragia vaginal.
Esta hemorragia puede aparecer incluso en chicas que aún no han tenido el primer periodo menstrual, si accidentalmente han tomado este medicamento.
Si usted ha tomado demasiados comprimidos de aidrana, o descubre que un niño los ha tomado, consulte con su médico o farmacéutico.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
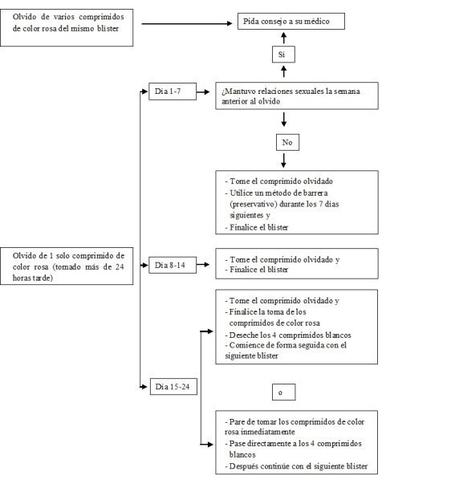
Si olvidó tomaraidrana
Los 4 últimos comprimidos de la 4ª fila del blíster son comprimidos de placebo. Si usted olvidó uno de estos comprimidos, no se pierde efecto anticonceptivo de aidrana. Debe desechar el comprimido placebo olvidado.
Si usted olvidó un comprimido activo de color rosa (comprimidos 1–24 del blíster), debe seguir los siguientes consejos:
- Si usted se retrasa menos de 24 horasen la toma de algún comprimido, la protección frente al embarazo no disminuye. Tome el comprimido tan pronto como se acuerde y los comprimidos siguientes a la hora habitual.
- Si usted se retrasa más de 24 horasen la toma de algún comprimido, la protección frente al embarazo puede reducirse. Cuanto más comprimidos haya olvidado, mayor es el riesgo de tener un embarazo.
El riesgo de protección incompleta frente al embarazo es máximo si usted olvida tomar un comprimido de color rosa al principio o al final del blíster. A continuación se enumeran las recomendaciones a seguir en esta situación (véase también el diagrama más abajo):
- Olvido de más de un comprimido del blíster
Consulte con su médico.
- Olvido de un comprimido durantelos días 1–7 (primera fila)
Tome el comprimido olvidado tan pronto como se acuerde, aunque esto signifique que tenga que tomar dos comprimidos a la vez. Siga tomando los comprimidos siguientes a la hora habitual y utilice precauciones adicionalespor ejemplo, preservativos, durante los 7 días siguientes. Si usted ha mantenido relaciones sexuales en la semana previa al olvido del comprimido debe saber que hay un riesgo de embarazo. En ese caso, consulte con su médico.
- Olvido de un comprimido durante los días 8–14 (segunda fila)
Tome el comprimido olvidado tan pronto como se acuerde, aunque esto signifique que tenga que tomar dos comprimidos a la vez. Siga tomando los comprimidos siguientes a la hora habitual. La protección frente al embarazo no disminuye y usted no necesita tomar precauciones adicionales.
- Olvido de un comprimido entre los días 15 y 24 (tercera o cuarta fila)
Puede elegir entre dos posibilidades:
- Tome el comprimido olvidado tan pronto como se acuerde, aunque esto signifique que tenga que tomar dos comprimidos a la vez. Siga tomando los comprimidos siguientes a la hora habitual. En lugar de continuar con los comprimidos blancos de placebo, deséchelos y comience a tomar el siguiente blíster (el día en que toma el primer comprimido será diferente).
Probablemente, tendrá la regla al final del segundo blíster –durante la toma de los comprimidos blancos de placebo– aunque puede presentar manchado o hemorragia parecida a la menstruación durante la toma del segundo blíster.
- También puede interrumpir la toma de comprimidos activos de color rosa y pasar directamente a los 4 comprimidos blancos de placebo (antes de tomar los comprimidos de placebo, anote el día en el que olvidó tomar su comprimido). Si quiere comenzar un nuevo blíster en su día fijado de inicio, tome los comprimidos de placebo durante menos de 4 días.
Si usted sigue una de estas dos recomendaciones, permanecerá protegida frente al embarazo.
Si usted ha olvidado tomar algún comprimido de un blíster y no tiene la regla durante los días de placebo, esto puede significar que está embarazada. En este caso, debe acudir a su médico antes de seguir con el siguiente blíster.
Qué debe hacer en caso de vómitos o diarrea intensa
Si usted tiene vómitos en las 3–4 horas siguientes a la toma de un comprimido activo de color rosa o si padece diarrea intensa, hay riesgo de que los principios activos del comprimido no sean absorbidos totalmente por su organismo. Esto es similar a lo que ocurre cuando usted olvida un comprimido. Tras los vómitos o la diarrea, debe tomar un comprimido de color rosa de un envase de reserva lo antes posible. Si es posible, tómelo dentro de las 24 horas posteriores a la hora habitual en que toma su anticonceptivo. Si no es posible o han transcurrido más de 24 horas, siga los consejos de la sección “Si olvidó tomar aidrana”.
Retraso del periodo menstrual: qué debe saber
Aunque no es recomendable, es posible retrasar su periodo menstrual si no toma los comprimidos blancos de placebo de la 4ª fila y comienza directamente a tomar los comprimidos de un nuevo blíster de aidrana hasta finalizar este nuevo blíster. Usted puede experimentar manchado (gotas o manchas de sangre) o hemorragia parecida a la menstruación durante el uso del segundo blíster. Tras finalizar este segundo blíster tomando los 4 comprimidos blancos de la 4ª fila, se inicia el siguiente blíster.
Antes de decidir el retraso de su periodo menstrual, pregunte a su médico.
Cambio del primer día del periodo menstrual: qué debe saber
Si usted toma los comprimidos según las instrucciones, su periodo menstrual comenzará durante los días de placebo. Si usted tiene que cambiar este día, lo puede hacer reduciendo los días de placebo (los días en los que toma los comprimidos blancos) ¡pero nunca alargando – 4 días es el máximo! Por ejemplo, si empieza los días de placebo un viernes y quiere cambiarlo a martes (3 días antes), debe comenzar un nuevo blíster 3 días antes de lo habitual. Es posible que no se produzca hemorragia durante estos días de placebo. Entonces usted puede experimentar manchados o hemorragia parecida a la menstruación.
Si no está segura de cómo proceder, consulte con su médico.
Si interrumpe el tratamiento conaidrana
Usted puede dejar de tomar aidrana siempre que lo desee. Si no quiere quedarse embarazada, consulte con su médico acerca de otros métodos eficaces de control de la natalidad. Si quiere quedarse embarazada, deje de tomar aidrana y espere hasta su periodo menstrual antes de intentar quedarse embarazada. Así podrá calcular la fecha estimada del parto más fácilmente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si sufre cualquier efecto adverso, especialmente si es grave y persistente, o tiene algún cambio de salud que cree que puede deberse a aidrana, consulte a su médico.
Contacte con un médico de forma inmediata si experimenta cualquiera de los siguientes síntomas de angioedema: hinchazón de la cara, lengua y/o garganta, y/o dificultad para tragar o urticaria con posible dificultad para respirar (ver también sección “Advertencias y precauciones”).
Todas las mujeres que toman anticonceptivos hormonales combinados corren mayor riesgo de presentar coágulos de sangre en las venas (tromboembolismo venoso (TEV)) o coágulos de sangre en las arterias (tromboembolismo arterial (TEA)). Para obtener información más detallada sobre los diferentes riesgos de tomar anticonceptivos hormonales combinados, ver sección 2 “Qué necesita saber antes de empezar a tomar aidrana”.
A continuación se describen los efectos adversos relacionados con el uso de aidrana:
Efectos adversos frecuentes: pueden afectar hasta 1 de cada 10 personas
- cambios en el estado de ánimo
- dolor de cabeza
- náuseas
- dolor de las mamas, problemas con los periodos como periodos irregulares, ausencia de periodos
Efectos adversos poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- depresión, nerviosismo, somnolencia
- mareo, hormigueos y pinchazos
- migraña, venas varicosas, aumento de la presión arterial
- dolor de estómago, vómitos, indigestión, gases intestinales, inflamación del estómago, diarrea
- acné, picor, erupción cutánea
- molestias y dolores, como dolor de espalda, dolor en las extremidades, calambres musculares
- infección vaginal por hongos, dolor en la región abdominal inferior (pélvica), aumento de tamaño de las mamas, bultos benignos en las mamas, hemorragias uterino/vaginales (que suelen remitir a lo largo del tratamiento), secreción vaginal, sofocos, inflamación de la vagina (vaginitis), problemas con las reglas, reglas dolorosas, reglas más cortas, reglas abundantes, sequedad vaginal, frotis cervical anormal, pérdida de interés por el sexo
- falta de energía, aumento de la sudoración, retención de líquidos
- aumento de peso
Efectos adversos raros: pueden afectar hasta 1 de cada 1.000 personas
- cándida (una infección por hongos)
- anemia, aumento del número de plaquetas en la sangre
- reacción alérgica
- trastorno hormonal (endocrino)
- aumento del apetito, pérdida del apetito, concentración de potasio en la sangre anormalmente elevada, concentración de sodio en la sangre anormalmente baja
- ausencia de orgasmo, insomnio
- vértigo, temblores
- trastornos oculares, como inflamación de los párpados, sequedad ocular
- frecuencia del corazón inusualmente rápida
- inflamación de una vena, sangrado nasal, desvanecimiento
- aumento de tamaño del abdomen, trastorno intestinal, sensación de flatulencia, hernia gástrica, infección de la boca por hongos, estreñimiento, sequedad de boca
- dolor en los conductos biliares o vesícula biliar, inflamación de la vesícula biliar
- manchas pardo amarillentas en la piel, eczema, pérdida de pelo, inflamación de la piel parecida al acné, sequedad de piel, inflamación de la piel con hinchazones, crecimiento excesivo del pelo, trastornos de la piel, marcas de tensión sobre la piel, inflamación de la piel, inflamación de la piel por fotosensibilidad, nódulos de la piel
- relaciones sexuales difíciles o dolorosas, inflamación de la vagina (vulvovaginitis), hemorragias después de las relaciones sexuales, hemorragia por privación, quiste en las mamas, aumento del número de células mamarias (hiperplasia), bultos malignos en las mamas, crecimiento anormal de la superficie mucosa del cuello del útero, encogimiento o pérdida del recubrimiento del útero, estructuras saculares llenas de líquido en un ovario, aumento de tamaño del útero
- malestar
- pérdida de peso
- coágulos de sangre perjudiciales en una vena o arteria, por ejemplo:
- En una pierna o pie (es decir, TVP).
- En un pulmón (es decir, EP).
- Ataque al corazón.
- Ictus.
- Ictus leve o síntomas temporales similares a los de un ictus, lo que se llama accidente isquémico transitorio (AIT).
- Coágulos de sangre en el hígado, estómago/intestino, riñones u ojo
Las posibilidades de tener un coágulo de sangre pueden ser mayores si tiene cualquier otra afección que aumente este riesgo (ver sección 2 para obtener más información sobre las afecciones que aumentan el riesgo de padecer coágulos de sangre y los síntomas de un coágulo de sangre).
También se han comunicado los siguientes efectos adversos, pero su frecuencia no puede estimarse a partir de los datos disponibles: hipersensibilidad, eritema multiforme (erupción cutánea con rojeces en forma de diana o úlceras).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de aidrana
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30 ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de aidrana
- Los principios activos son drospirenona y etinilestradiol.
Cada comprimido recubierto con película activo de color rosa contiene 3 miligramos de drospirenona y 0,02 miligramos de etinilestradiol.
Los comprimidos recubiertos con película de color blanco no contienen principio activo.
- Los demás componentes son:
Comprimidos recubiertos con película activos de color rosa
Núcleo del comprimido:lactosa monohidrato, polacrilín potásico, povidona K-30, estearato de magnesio.
Recubrimiento con película del comprimido:macrogol 3350, dióxido de titanio (E171), poli (alcohol) vinílico, talco, óxido de hierro rojo (E172) y óxido de hierro amarillo (E172).
Comprimidos recubiertos con película inactivos blancos:
Núcleo del comprimido:lactosa monohidrato, polacrilín potásico, povidona K-30, estearato de magnesio, sílice coloidal anhidra
Recubrimiento con película del comprimido:macrogol 3350, dióxido de titanio (E171), poli (alcohol) vinílico, talco.
Aspecto del producto y contenido del envase
- Cada blíster de aidrana contiene 24 comprimidos recubiertos con película activos de color rosa en la 1ª, 2ª, 3ª y 4ª fila del blíster, y 4 comprimidos recubiertos con película blancos de placebo en la 4ª fila.
- Los comprimidos de aidrana tanto de color rosa como blancos, son comprimidos recubiertos con película; el núcleo del comprimido está recubierto.
- El comprimido activo es cilíndrico con un diámetro de 6 mm aproximadamente, biconvexo y de color rosa.
- El comprimido placebo es cilíndrico con un diámetro de 6 mm aproximadamente, biconvexo y de color blanco.
aidrana está disponible en envases de 1 y 3 blísteres, cada uno con 28 comprimidos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Títular de la autorización de comercialización
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) - España
Responsable de la fabricación
Cyndea Pharma, S.L.
Polígono Industrial Emiliano Revilla
Av. De Ágreda 31
42110 Ólvega (Soria)- España
o
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) - España
Fecha de la última revisión de este prospecto:julio 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en prospecto y cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/83761/P_83761.html
Código QR a: https://cima.aemps.es/cima/dochtml/p/83761/P_83761.html
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a AIDRANA 3 MG/0,02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFGForma farmacéutica: COMPRIMIDO, 3 mg/0,03 mgPrincipio activo: drospirenone and ethinylestradiolFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 3 mg/0,03 mgPrincipio activo: drospirenone and ethinylestradiolFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 3 mg/0,02 mgPrincipio activo: drospirenone and ethinylestradiolFabricante: Laboratorios Cinfa S.A.Requiere receta
Médicos online para AIDRANA 3 MG/0,02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de AIDRANA 3 MG/0,02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






