
GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO

Como usar GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o utilizador
GAMMAGARD S/D 5 g, pó e dissolvente para solução para perfusão
Imunoglobulina humana normal
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é GAMMAGARD S/D e para que é utilizado
- O que precisa saber antes de começar a usar GAMMAGARD S/D
- Como usar GAMMAGARD S/D
- Possíveis efeitos adversos
- Conservação de GAMMAGARD S/D
- Conteúdo do envase e informação adicional
1. O que é GAMMAGARD S/D e para que é utilizado
GAMMAGARD S/D pertence a uma classe de medicamentos chamados imunoglobulinas. Estes medicamentos contêm anticorpos humanos, presentes também no sangue. Os anticorpos ajudam a combater as infecções. Os medicamentos como GAMMAGARD S/D são utilizados quando não têm anticorpos suficientes no sangue. Estes pacientes costumam sofrer de infecções frequentes. GAMMAGARD S/D também pode ser utilizado quando se necessitam anticorpos adicionais para a cura de determinados distúrbios inflamatórios (doenças autoimunes).
GAMMAGARDS/D5 g é utilizado para
Tratamento de pacientes que não têm anticorpos suficientes (tratamento de reposição). Há cinco grupos:
- Pacientes com uma falta congênita de produção de anticorpos (síndromes de imunodeficiência primária (IDP)) como:
- agammaglobulinemia ou hipogammaglobulinemia congênitas,
- imunodeficiência comum variável,
- imunodeficiências combinadas graves,
- Síndrome de Wiskott-Aldrich
- Pacientes com um cancro do sangue (leucemia linfocítica crónica) que provoque uma falta de produção de anticorpos e infecções recorrentes quando o tratamento preventivo com antibióticos tenha falhado.
- Pacientes com um cancro da medula óssea (mieloma múltiplo) e falta de produção de anticorpos com infecções recorrentes nos quais a resposta à vacina contra determinadas bactérias (neumococos) tenha falhado.
- Crianças e adolescentes (0 a 18 anos) com SIDA de nascimento e infecções frequentes.
- Pacientes com uma baixa produção de anticorpos após um transplante de células da medula óssea de outra pessoa.
Tratamento de pacientes com determinados distúrbios inflamatórios (efeito imunomodulador). Há três grupos:
- Pacientes que não têm suficientes plaquetas no sangue (púrpura trombocitopénica idiopática/primária, PTI) e com um alto risco de hemorragia ou que vão ser submetidos a uma intervenção cirúrgica próximamente.
- Pacientes com uma doença que provoca a inflamação múltipla de diversos órgãos do corpo (Doença de Kawasaki).
- Pacientes com uma doença que se caracteriza pela inflamação múltipla dos nervos de todo o corpo (Síndrome de Guillain Barré).
2. O que precisa saber antes de começar a usar GAMMAGARD S/D
Não useGAMMAGARDS/D
- Se é alérgico (hipersensível) às imunoglobulinas ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- Se tem uma deficiência de imunoglobulina A. Pode ter anticorpos anti-imunoglobulina A no sangue. GAMMAGARD S/D contém quantidades muito pequenas de imunoglobulina A e pode desenvolver uma reação alérgica.
Advertências e precauções
Período de controlo requerido durante a perfusão
- Vai ser controlado atentamente durante o período de perfusão de GAMMAGARD S/D para evitar que sofra uma reação alérgica. O seu médico se asegurará de que a velocidade de perfusão de GAMMAGARD S/D é a adequada no seu caso.
Pode existir um risco maior de efeitos adversos:
- se GAMMAGARD S/D for administrado a uma velocidade alta,
- se sofrer de um distúrbio que se caracteriza por um nível baixo de anticorpos no sangue (hipo- ou agammaglobulinemia),
- se não tiver recebido este medicamento antes ou
- se tiver passado um longo período (por exemplo, várias semanas) desde a última vez que lhe foi administrado.
Nestes casos, vai ser controlado estreitamente durante a perfusão e durante uma hora após o término da perfusão, pois pode existir um risco maior de efeitos adversos.
Se já tiver recebido GAMMAGARD S/D recentemente, apenas será observado durante a perfusão e durante, pelo menos, 20 minutos após a perfusão.
Quando é necessário deter ou diminuir a velocidade da perfusão
Em casos raros, o seu organismo pode estar sensibilizado a medicamentos que contêm anticorpos. Isso pode ocorrer sobretudo se apresentar uma deficiência de imunoglobulina A. Nesses casos raros, podem sofrer de reações alérgicas como um descenso brusco da tensão arterial ou um choque, mesmo que tenha recebido anteriormente um tratamento com medicamentos que contêm anticorpos.
- Se notar algum dos sintomas seguintes, informe imediatamente o seu médico ou enfermeira:
- Sibilâncias repentinas, dificuldade em respirar ou opressão no peito
- Dor de cabeça
- Febre
- Inchaço dos párpados, face, lábios ou vasos sanguíneos
- Bolhas ou manchas vermelhas com picazón na pele
- Picazón por todo o corpo
Dependendo da decisão do seu médico, pode ser necessário diminuir a velocidade ou parar a perfusão.
Grupos de pacientes especiais
O seu médico deve ter precauções se sofrer de excesso de peso, for de idade avançada, for diabético, estiver imobilizado, se utilizar estrógenos, tiver uma sonda vascular permanente ou tiver tendência a sofrer de tromboses.
O seu médico vai observá-lo cuidadosamente se tiver:
- tensão arterial alta
- o volume de sangue baixo (hipovolemia)
- aumento da viscosidade do sangue ou problemas nos vasos sanguíneos (doenças vasculares, incluindo gasto cardíaco ou episódios trombóticos)
- coagulação excessiva ou distúrbios de coagulação.
Nestes casos, as imunoglobulinas podem aumentar o risco de infarto do miocárdio, acidente cerebrovascular, embolia pulmonar ou trombose venosa profunda, embora em casos muito raros.
Informe o seu médico se for diabético.
Este medicamento contém glicose. GAMMAGARD S/D não contém sacarose nem maltose.
Os pacientes com diabetes mellitus devem ter em conta que uma solução a 5% (50 mg/ml) de GAMMAGARD S/D contém 400 mg de glicose por grama de IgG. Um paciente de 70 kg que recebe uma dose de 1 g/kg de IgG receberia 28 gramas de glicose, o que pode afetar o seu nível de açúcar no sangue.
O seu médico também terá um cuidado especial
- se tiver ou tiver tido problemas com os rins
- se receber medicamentos que possam danificar os rins (medicamentos nefrotóxicos), pois existe uma probabilidade muito rara de falha renal aguda. Informe o seu médico se tiver ou tiver tido problemas renais.
O conteúdo de proteínas pode aumentar, provocando uma maior viscosidade do sangue
Informação sobre o material original deGAMMAGARD S/D
GAMMAGARD S/D é fabricado a partir de plasma humano (a parte líquida do sangue). Quando os medicamentos são elaborados a partir de sangue ou plasma humanos, devem ser adotadas um número de medidas para prevenir uma possível transmissão de infecções aos pacientes. Estas medidas incluem uma seleção cuidadosa dos doadores de sangue e plasma para garantir a exclusão de doadores com risco de sofrer de infecções e a análise de cada doação e mistura de plasmas para detectar possíveis vírus ou infecções. Os fabricantes de produtos incluem, além disso, uma série de etapas no processamento do sangue ou do plasma que podem inativar ou eliminar os vírus. Apesar destas medidas, quando se administram medicamentos preparados a partir de sangue ou plasma humanos, não se pode excluir totally a possibilidade de transmissão de infecções. Isso é aplicável também a vírus desconhecidos ou emergentes e a outros tipos de infecções.
As medidas adotadas são consideradas eficazes para os vírus encapsulados, como o vírus da imunodeficiência humana (VIH), o vírus da hepatite B (VHB) e o vírus da hepatite C (VHC), e para o vírus não encapsulado da hepatite A (VHA). As medidas adotadas podem ter um valor limitado para vírus não encapsulados, como o parvovirus B19.
As imunoglobulinas não se associaram a infecções por hepatite A ou parvovirus B19, possivelmente porque os anticorpos contra estas infecções, que estão presentes no produto, são protectores.
Recomenda-se que cada vez que se lhe administre GAMMAGARD S/D, se deixe constância do nome do medicamento e número de lote administrado para manter um registo dos lotes utilizados.
Uso de GAMMAGARD S/D com outros medicamentos
Comunique ao seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento ou se foi vacinado nas últimas seis semanas.
A perfusão de imunoglobulinas, como GAMMAGARD S/D, pode alterar a eficácia de algumas vacinas de vírus vivos, como a do sarampo, rubéola, papeira e varicela. Por isso, após a administração destes medicamentos, pode ter que esperar até 3 meses antes de receber uma vacina de vírus vivo atenuado. Pode ter que esperar até 1 ano após receber imunoglobulinas antes da administração da vacina contra o sarampo.
Efeitos sobre os análises de sangue
GAMMAGARD S/D contém uma ampla variedade de anticorpos diferentes, alguns dos quais podem interferir com os análises de sangue. Se lhe for realizado um análise de sangue, por favor informe o analista ou o seu médico de que lhe foi administrado GAMMAGARD S/D.
A administração de Gammagard S/D pode dar origem a leituras de falsos positivos nas provas que dependem da detecção de beta-D-glucanos para o diagnóstico de infecções fúngicas; isso pode perdurar durante as semanas seguintes à perfusão do produto.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
- Não foram realizados ensaios clínicos com GAMMAGARD S/D em mulheres grávidas ou em período de lactação. Os anos de experiência clínica com medicamentos que contêm anticorpos demonstraram que não devem ser esperados efeitos prejudiciais durante a gravidez nem para o filho.
- Se está em período de lactação, os anticorpos de GAMMAGARD S/D podem ser encontrados no leite materno. Por isso, o seu bebê pode estar protegido contra certas infecções.
- Os efeitos de GAMMAGARD S/D sobre a fertilidade não foram estabelecidos.
Condução e uso de máquinas
Os pacientes podem experimentar reações (por exemplo, tontura ou náuseas) durante o tratamento com GAMMAGARD S/D que possam afetar a capacidade de conduzir e utilizar máquinas.
Se isso ocorrer, espere até que as reações tenham desaparecido.
Gammagard S/D 5 g contém sódio e glicose
Este medicamento contém 334 mg de sódio (componente principal da sal de mesa/para cozinhar) em cada frasco. Isso equivale a 17% da ingestão diária máxima de sódio recomendada para um adulto.
Este medicamento contém glicose. Os pacientes com diabetes mellitus devem ter em conta que uma solução a 5% (50 mg/ml) de GAMMAGARD S/D contém 400 mg de glicose por grama de IgG. Um paciente de 70 kg que recebe uma dose de 1 g/kg de IgG receberia 28 gramas de glicose, o que pode afetar o seu nível de açúcar no sangue.
3. Como usar GAMMAGARD S/D
GAMMAGARD S/D é para administração intravenosa (injeção na veia). Ser-lhe-á administrado pelo seu médico ou enfermeira. A dose e a frequência da perfusão podem variar dependendo da sua situação e peso corporal.
No início da perfusão, receberá GAMMAGARD S/D a uma velocidade baixa. O seu médico pode aumentar gradualmente a velocidade de perfusão dependendo de se tolera bem.
Uso em crianças
Em crianças (0 a 18 anos) são utilizadas as mesmas indicações, doses e frequência de perfusão que nos adultos.
Se usar maisGAMMAGARDS/Ddo que deve
Se receber mais GAMMAGARD S/D do que devia, o sangue pode espessar (hiperviscosidade). Quanto mais espesso for o sangue, mais difícil é o seu transporte através dos vasos do seu organismo, por isso, será transportada menos quantidade de oxigénio para os órgãos vitais, como o cérebro, pulmões, etc. Isso pode ocorrer sobretudo se for um paciente de risco (por exemplo, um paciente mais velho ou um paciente com problemas renais ou cardíacos). Certifique-se de tomar os líquidos adequados para não desidratar e informe o seu médico se tiver problemas de saúde.
Em caso de sobredose ou administração acidental, consulte o Serviço de Informação Toxicológica. Telefone 915 620 420.
4. Possíveis efeitos adversos
Como todos os medicamentos, GAMMAGARD S/D pode produzir efeitos adversos, embora nem todas as pessoas os sofram. No entanto, os possíveis efeitos adversos podem ser reduzidos ao diminuir a velocidade de perfusão.
Os seguintes efeitos adversos podem ocorrer geralmente após o tratamento com imunoglobulinas (medicamentos como GAMMAGARD S/D):
- Frequentes (podem afetar até 1 de cada 10 pessoas): arrepios, dor de cabeça, febre, vómitos, náuseas.
- Pouco frequentes (podem afetar até 1 de cada 100 pessoas): leve dor na parte inferior das costas.
- Raros (podem afetar até 1 de cada 1000 pessoas): casos de descenso brusco da tensão arterial, sintomas semelhantes ao eczema (reações cutâneas transitórias).
- Frequência não conhecida (não pode ser estimada a partir dos dados disponíveis): reações alérgicas, mesmo em pacientes que não apresentaram reações às perfusões anteriores; inflamação temporária das membranas do cérebro (meningite asséptica reversível); redução temporária do número de glóbulos vermelhos no sangue; aumentos transitórios dos valores da função hepática (transaminases) e aumento do conteúdo de creatinina no sangue e falha renal; formação de coágulos sanguíneos nas veias que tiveram como resultado um ataque cardíaco, acidente cerebrovascular, dano nos pulmões e trombose venosa profunda; dor nas articulações; tensão arterial baixa.
A seguir são descritos os efeitos adversos que alguns pacientes relataram com GAMMAGARD S/D nos ensaios clínicos e os efeitos adversos relatados durante a experiência pós-comercialização:
- Frequentes (podem afetar até 1 de cada 10 pessoas): dor de cabeça, rubor, náuseas, vómitos, fadiga, arrepios, febre.
- Pouco frequentes (podem afetar até 1 de cada 100 pessoas): gripe, ansiedade, agitação, sonolência anormal, visão borrosa, sentir os batimentos do coração, dificuldade em respirar, sangramento nasal, diarreia, dor na parte superior do abdômen, mal-estar de estômago, inflamação da boca, picazón, erupções cutâneas, suor frio, excesso de sudorese, dor nas costas, cãibras musculares, dor nos braços e pernas, dor no peito, mal-estar no peito, sensação anormal, sensação de frio, sensação de calor, sintomas semelhantes aos da gripe, rubor no local da injeção, saída do medicamento da via no local da injeção, dor no local da injeção, sensação de náuseas/vómitos, dor, tensão arterial alta, alterações da tensão arterial, perda de apetite.
- Frequência não conhecida (não pode ser estimada a partir dos dados disponíveis): inflamação das membranas do cérebro não causada por uma infecção bacteriana, destruição de glóbulos vermelhos, diminuição da quantidade de glóbulos vermelhos, diminuição da quantidade de plaquetas, inflamação dos nódulos linfáticos, reações alérgicas de todo o tipo de gravidade, como choque alérgico, nervosismo, tontura, sensação anormal na pele, tremor involuntário, ataques, hemorragia cerebral, acidente cerebrovascular (transitório), enxaqueca, perda de consciência, intolerância à luz, alteração visual, dor no olho, oclusão do vaso sanguíneo central do olho, ataque cardíaco, coloração azulada da pele, aumento do ritmo cardíaco, diminuição do ritmo cardíaco, tensão arterial alta, palidez, tensão arterial baixa, inflamação das veias, oclusão dos vasos sanguíneos, tosse, opressão de garganta, diminuição do nível de oxigénio no sangue, hiperventilação, pitidos no peito, espasmos nas vias respiratórias, oclusão dos vasos sanguíneos dos pulmões, líquido nos pulmões, alteração da digestão, dor abdominal, inflamação do fígado (não transmissível), rubor da pele, erupções cutâneas, inflamação da pele, inflamação alérgica das camadas profundas da pele, dor muscular e das articulações, falha renal, fraqueza generalizada, inchaço dos tecidos do corpo, reações no local da injeção e perfusão, resultado positivo do teste de Coombs.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es.
Ao comunicar efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de GAMMAGARD S/D
- Mantenha fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece no envase após CAD. A data de validade é o último dia desse mês.
- Não use se observar partículas ou decoloração.
- Não conserve a uma temperatura superior a 25ºC.
- Não congele.
Mantenha o envase no embalagem exterior para protegê-lo da luz.
6. Conteúdo do envase e informação adicional
Composição deGAMMAGARD S/D
O princípio ativo de GAMMAGARD S/D é imunoglobulina humana normal.
GAMMAGARD S/D pode ser reconstituído com água esterilizada para preparações injetáveis como uma solução de proteína a 5 % (50 mg/ml) ou a 10 % (100 mg/ml). Pelo menos, 90% é imunoglobulina G (IgG).
Os demais componentes são albumina humana, glicina, cloreto de sódio e glicose monohidrato.
Aspecto do produto e conteúdo do envase
GAMMAGARD S/D é um pó liofilizado de cor branca ou ligeiramente amarela, substancialmente livre de partículas estranhas visíveis. GAMMAGARD S/D está disponível em envases de 5 g e 10 g.
Cada envase contém
- um frasco de pó de 5 g
- 96 ml de água para preparações injetáveis
- um dispositivo estéril de transferência
- um equipamento estéril de administração com filtro
Título da autorização de comercialização e responsável pela fabricação:
Título da autorização de comercialização:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Viena
Áustria
Responsável pela fabricação:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart, 80 (Lessines)
B-7860-Bélgica
Representante local:
Takeda Farmacêutica España S.A.
Rua Albacete, 5, 9º andar,
Edifício Los Cubos
28027 Madrid
Espanha
Tel: +34 91 790 42 22
Este prospecto foi aprovado em Janeiro de 2021
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------------
ESTA INFORMAÇÃO ESTÁ DESTINADA ÚNICAMENTE A MÉDICOS OU PROFISSIONAIS DO SETOR SANITÁRIO
Precauções especiais durante a conservação
Foi demonstrada uma estabilidade em uso química e física de GAMMAGARD S/D reconstituído de 24 horas a temperatura ambiente. Desde o ponto de vista microbiológico, o produto deve ser utilizado imediatamente, o tempo e as condições de conservação antes do uso são responsabilidade do usuário e normalmente não devem ser superiores a 24 horas a 2-8ºC, quando a reconstituição se tenha realizado em condições assépticas controladas e validadas.
Reconstituição: Usar uma técnica asséptica:
Depois da reconstituição, apenas se administrarão soluções transparentes ou ligeiramente opalescentes e incolores ou amareladas.
Levar o frasco de pó e o frasco de água para preparações injetáveis (solvente) a temperatura ambiente. Manter esta temperatura até completar a dissolução.
- Solução a 5%
- Retirar os protetores dos frascos e limpar os tampões com solução germicida.
- Retirar o protetor que cobre o punção do dispositivo de transferência. Não tocar o punção.
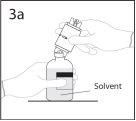
3a. Colocar o frasco de solvente sobre uma superfície lisa.
Utilizar o extremo do punção que fica ao descoberto para
perfurar o frasco de solvente através do centro do tampão.
PRECAUÇÃO: se não se introduzir o punção no centro do
tampão, este pode soltar-se e perder-se o vácuo.
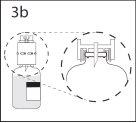
3b. Asegurar que o pescoço do frasco fica encaixado totalmente no
dispositivo pressionando firmemente o dispositivo de transferência.
Retirar o protetor que cobre o outro extremo do punção enquanto
se segura o dispositivo de transferência. Não tocar o punção.
- Manter o frasco de solvente com o dispositivo de transferência conectado em um ângulo com respeito ao frasco de pó para prevenir que o solvente saia.
Nota: não colocar o frasco de solvente para baixo, pois o solvente pode derramar-se.
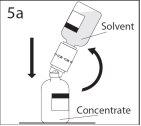 5a. Perfurar o frasco de pó através do centro do
5a. Perfurar o frasco de pó através do centro do
tampão enquanto que se inverte rapidamente o frasco de solvente
para evitar que o solvente saia.
PRECAUÇÃO: se não se introduzir o punção no
centro do tampão, este pode soltar-se e perder-se o
vácuo.
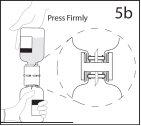 5b. Asegurar que o pescoço do frasco fica encaixado totalmente
5b. Asegurar que o pescoço do frasco fica encaixado totalmente
no dispositivo pressionando firmemente o frasco de solvente.
- Depois de que todo o solvente tenha passado ao
frasco de pó, retirar o dispositivo de transferência e o frasco
de solvente vazio. Girar imediatamente o frasco de concentrado para misturar totalmente o conteúdo.
PRECAUÇÃO: não agitar. Evitar a formação de espuma.
Depois de um único uso, descartar o dispositivo de transferência.
- Solução a 10%
- Retirar os protetores dos frascos e limpar os tampões com solução germicida.
- Para preparar uma solução a 10% é necessário extrair a metade do volume do solvente. A tabela 2 descreve o volume de solvente que se deve extrair de cada frasco para conseguir uma solução a 10% antes de conectar o dispositivo de transferência. Utilizando uma técnica asséptica, retirar o volume não necessário de solvente utilizando uma seringa hipodérmica estéril e uma agulha. Descartar a seringa e a agulha que contêm a quantidade de solvente não requerida.
- Utilizando o solvente residual no frasco do solvente, seguir os passos 2-6 descritos na seção A.
TABELA 2
5 g
Concentração frasco
5% Para a reconstituição a 5% não extrair nenhuma quantidade de solvente
10% 48 ml
Administração. Usar uma técnica asséptica
Seguir as instruções de uso do folheto que acompanha o equipamento de administração incluído no envase. Se se utilizar outro equipamento de administração, asegurar-se de que contém um filtro similar.
Instruções de uso e manipulação
O produto deve ser levado a temperatura ambiente ou temperatura corporal antes de seu uso.
A dissolução completa deve ser conseguida em 30 minutos.
A solução resultante deve ser transparente ou ligeiramente opalescente e incolora ou amarelada. Não utilizar soluções que estejam turvas ou contenham sedimentos. O produto reconstituído deve ser inspecionado visualmente antes de sua administração para verificar a ausência de partículas e coloração.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele, será realizada de acordo com as normas locais.
Eliminar o equipamento de transferência após seu único uso.
Forma de administração
Via intravenosa.
Se possível, se recomenda que a solução a 10% de GAMMAGARD S/D seja administrada através das veias antecubitais. Isso pode reduzir a probabilidade de que se produzam desconfortos no local da perfusão.
GAMMAGARD S/D a 5% (50 mg/ml) deve ser administrado por via intravenosa a uma velocidade inicial de 0,5 ml/kg/h. Em geral, se recomenda que os pacientes que recebam GAMMAGARD S/D pela primeira vez ou que mudem de outra imunoglobulina intravenosa para GAMMAGARD S/D, iniciem o tratamento com a velocidade de administração mais baixa e luego aumentem a velocidade máxima, se previamente tenham tolerado várias perfusões a velocidades de perfusão intermédias.
Se se tolera bem, a velocidade de administração da solução a 5% pode ser aumentada gradualmente até um máximo de 4 ml/kg/h. Quando se muda de uma solução a 5% para uma solução a 10%, a velocidade de administração da solução a 10% deve ser inicialmente baixa para manter comparável a velocidade de administração da proteína IgG. Em muitos pacientes é possível aumentar gradualmente a velocidade de administração da solução a 10% até 8 ml/kg/h. A velocidade de administração será ajustada individualmente de acordo com a tolerabilidade do paciente.
Precauções especiais
Qualquer efeito adverso relacionado com a perfusão deve ser tratado reduzindo a velocidade ou parando a perfusão.
Cada vez que se administra GAMMAGARD S/D se recomenda indicar o nome e o número de lote do produto.
Incompatibilidades
GAMMAGARD S/D não deve ser misturado com outros medicamentos. Se recomenda administrar GAMMAGARD S/D de forma separada a outros medicamentos que o paciente receba.
Dosagem recomendada
INDICAÇÃO | DOSE | FREQUÊNCIA DE INJEÇÃO/PERFUSÃO |
Tratamento de reposição em imunodeficiência primária Tratamento de reposição em imunodeficiência secundária SIDA congênito Hipogamaglobulinemia (<4 g l) em pacientes que receberam um transplante< p> alogenético de células-mãe hematopoiéticas | Dose inicial: 0,4 – 0,8 g/kg Continuação: 0,2-0,8 g/kg 0,2-0,4 g/kg 0,2-0,4 g/kg 0,2-0,4 g/kg | a cada 3-4 semanas para obter um nível vale de IgG de, pelo menos, 5-6 g/l. a cada 3-4 semanas para obter um nível vale de IgG de, pelo menos, 5-6 g/l. a cada 3-4 semanas a cada 3-4 semanas para obter um nível vale de IgG acima de 5 g/l |
Imunomodulação: Trombocitopenia imune primária Síndrome de Guillain-Barré Doença de Kawasaki | 0,8-1 g/kg ou 0,4 g/kg /dia 0,4 g/kg/dia 1,6-2 g/kg ou 2 g/kg | no 1º dia, podendo ser repetido uma vez dentro dos três dias seguintes durante 2-5 dias durante 5 dias em várias doses durante 2-5 dias, juntamente com ácido acetilsalicílico em uma dose, juntamente com ácido acetilsalicílico. |
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mg/mlSubstância ativa: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mg/mlSubstância ativa: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mg/mlSubstância ativa: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requer receita médica
Alternativas a GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Polónia
Alternativa a GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Ukraine
Médicos online para GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de GAMMAGARD S/D 5 g, PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.














