FOSTIPUR KIT 300 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE


Cómo usar FOSTIPUR KIT 300 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fostipur Kit300 UIpolvo y disolvente para solución inyectable
Urofolitropina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fostipur Kit y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fostipur Kit
- Cómo usar Fostipur Kit
- Posibles efectos adversos
- Conservación de Fostipur Kit
- Contenido del envase e información adicional
1. Qué es Fostipur Kit y para qué se utiliza
- Fostipur Kit se utiliza para estimular la ovulación en mujeres que no ovulan y que no
responden a otros tratamientos (citrato de clomifeno).
- En la inducción del desarrollo multifolicular (y, por consiguiente, de varios óvulos) en
mujeres sometidas a tratamientos de fertilidad.
La urofolitropina es una hormona foliculostimulante altamente purificada que pertenece a un grupo de medicamentos llamados gonadotropinas.
Este medicamento debe utilizarse bajo control de su médico.
2. Qué necesita saber antes de empezar a usar Fostipur Kit
Antes de iniciar el tratamiento debe evaluarse la fertilidad de la pareja.
No use Fostipur Kit
- Si es alérgica a la urofolitropina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Aumento del tamaño de ovarios o quistes en el ovario sin ser consecuencia de un
trastorno hormonal (síndrome del ovario poliquístico).
- Hemorragia de origen desconocido.
- Cáncer de ovarios, uterino o de mama.
- Hinchazón anormal (tumor) de la glándula hipofisaria o hipotálamo (en el cerebro).
No debe utilizar este medicamento en caso de que tenga trastornos como menopausia prematura, malformación de los órganos sexuales o tumores propios de la matriz que le impidan que tenga lugar un embarazo normal.
Advertencias y precauciones
Aunque no se dispone de información sobre reacciones alérgicas con Fostipur Kit, debe comunicarle a su médico si tiene alguna reacción alérgica a medicamentos similares.
Este tratamiento aumenta el riesgo de desarrollar una enfermedad conocida como síndrome de hiperestimulación ovárica (SHO) (ver Posibles efectos adversos). Si se produce hiperestimulación ovárica, deberá interrumpir el tratamiento y evitará quedarse embarazada. Los primeros signos de la hiperestimulación ovárica son dolor en la región baja del abdomen, así como también, náuseas (malestar) vómitos y aumento de peso. Si aparecen estos síntomas, debe ser examinada por su médico lo antes posible. En casos graves, aunque raros, los ovarios pueden aumentar de tamaño y se puede acumular líquido en el abdomen o pecho.
El medicamento utilizado para lograr la liberación final de los óvulos maduros (que contiene la gonadotrofina coriónica humana, hCG) puede aumentar la probabilidad de padecer SHO. Por lo tanto, no es aconsejable utilizar hCG en los casos en que se está desarrollando una hiperestimulación ovárica y tampoco debería mantener relaciones sexuales incluso utilizando métodos anticonceptivos de barrera durante un mínimo de 4 días.
Debe tenerse en cuenta que las mujeres con problemas de fertilidad tienen un índice de abortos espontáneos superior al de la población normal.
La aparición de embarazos múltiples y nacimientos en pacientes que reciben tratamiento de inducción a la ovulación, aumenta si se compara con la concepción natural. Sin embargo, este riesgo puede reducirse si se utiliza la dosis recomendada.
Existe un ligero aumento del riesgo de embarazo extrauterino (embarazo ectópico) en mujeres con las trompas de Falopio dañadas.
Los embarazos múltiples y las características de los padres sometidos a tratamientos de fertilidad (por ejemplo, edad de la madre, características del esperma) pueden estar asociados con un mayor riesgo de anomalías en el nacimiento.
El tratamiento con Fostipur Kit, al igual que el embarazo en sí, puede aumentar el riesgo de padecer trombosis. La trombosis es la formación de un coágulo de sangre en un vaso sanguíneo, la mayoría de veces en las venas de las piernas o en los pulmones.
Consúltelo con su médico, antes de iniciar el tratamiento, especialmente:
- si usted ya sabe que tiene un riesgo mayor de padecer trombosis;
- si usted o un familiar próximo ha sufrido en alguna ocasión trombosis;
- si usted tiene un sobrepeso excesivo.
Este medicamento se prepara con orina de origen humano. El riesgo de transmisión de infección o enfermedad al organismo no se puede eliminar por completo. Sin embargo, este riesgo queda limitado por las fases de eliminación de virus en el proceso de fabricación, particularmente SIDA, Herpes virusy Papillomavirus.
No se han publicado casos de contaminación viral.
Uso de Fostipur Kit con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Fostipur Kit no debe ser utilizado si usted está embarazada o en período de lactancia.
Fostipur Kit contiene sodio
Este medicamento contiene menos de 1mmol de sodio (23 mg) por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Fostipur Kit
Dosis y duración del tratamiento:
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Mujeres que no ovulan y tienen menstruaciones irregulares o incompletas:
Si usted tiene la menstruación, el tratamiento debe iniciarlo dentro de los 7 días que siguen al inicio de la menstruación (los primeros 7 días del ciclo menstrual).
La dosis consiste en 1 inyección al día, bajo la piel (por vía subcutánea).
La dosis inicial común es de 75 UI hasta 150 UI de FSH (Fostipur Kit) al día. Esta dosis puede aumentarse si es necesario, de 37,5 hasta 75 UI en intervalos de 7 días o, preferiblemente, 14 días, para obtener una respuesta adecuada.
La dosis máxima diaria de FSH no debe exceder generalmente de 225 UI.
Si su médico no encuentra una respuesta adecuada tras 4 semanas de tratamiento, este ciclo de tratamiento debe interrumpirse. Para el siguiente ciclo, su médico le indicará un tratamiento con una dosis inicial más alta.
Cuando se obtenga una buena respuesta (crecimiento folicular satisfactorio), se le administrará únicamente una inyección de otro medicamento (hCG), utilizado para inducir la maduración folicular y la liberación de los óvulos. Esto tendrá lugar a las 24 - 48 horas después de la última inyección de Fostipur Kit. Se recomienda tener relaciones sexuales el mismo día de la administración de hCG y al día siguiente.
Si se obtiene una respuesta ovárica excesiva, el tratamiento debe interrumpirse y no se administrará hCG (ver Posibles efectos adversos). Para el siguiente ciclo, su médico le indicará una dosis inicial más baja.
Mujeres sometidas a estimulación ovárica por desarrollo folicular múltiple previo a fertilizaciónin vitrou otras técnicas de reproducción asistida:
Situación 1 – Si tiene la menstruación
El tratamiento debería empezarlo a los 2 ó 3 días del inicio de su menstruación (los primeros 2 ó 3 días del ciclo menstrual).
La dosis consiste en 1 inyección al día por vía subcutánea.
Una dosis habitualmente utilizada de superovulación consiste en la administración de 150 a 225 UI de Fostipur Kit, al día. El tratamiento prosigue, con el ajuste de la dosis según su respuesta, hasta alcanzar un desarrollo folicular adecuado. Éste se consigue generalmente al 10º día de tratamiento (un promedio de 5 a 20 días) y es evaluado mediante la toma de muestras sanguíneas y/o exámenes ecográficos.
La dosis máxima es, en general, de 450 UI/día.
Una vez logrado el desarrollo folicular, se administrará una sola inyección de un medicamento utilizado para inducir la maduración folicular final; este medicamento contiene hasta 10.000 UI de gonadotropina coriónica humana (hCG). Se administrará entre 24-48 horas después de la última inyección de Fostipur Kit.
La punción de los ovocitos se realizará aproximadamente unas 35 horas después.
Situación 2- Cuando se utiliza un agonista de la hormona liberadora de gonadotropina (GnRH)
Fostipur Kit deberá ser administrado aproximadamente 2 semanas después del inicio de este tratamiento. Ambos tratamientos se mantienen hasta alcanzar el desarrollo folicular adecuado. Se administrará una inyección de Fostipur Kit al día, por vía subcutánea. Por ejemplo, después de 2 semanas de tratamiento con un agonista de GnRH, se administrarán de 150 a 225 UI de Fostipur Kit durante los primeros 7 días. La dosis se ajustará entonces según la respuesta ovárica
Instrucciones de administración:
Fostipur Kit se administra por inyección bajo la piel (por vía subcutánea).
Cada vial es de un sólo uso y la inyección debe administrarse inmediatamente tras su preparación.
Tras aconsejarle y practicar convenientemente, su médico puede pedirle que se administre usted misma la inyección de Fostipur Kit.
En primer lugar, su médico debe:
- Dejarle practicar administrándose usted misma la inyección subcutánea.
- Indicarle cuáles son las zonas posibles para que usted se administre la inyección.
- Indicarle cómo preparar minuciosamente la solución para la inyección.
- Explicarle como preparar la dosis correcta que debe administrarse.
Antes de que usted se administre la inyección de Fostipur Kit, lea cuidadosamente las siguientes instrucciones:
Cómo preparar e inyectar 1 vial de Fostipur Kit, utilizando 1 vial de polvo:
La solución debe prepararse justo antes de ponerse la inyección. Cada vial se utiliza para un solo uso. El medicamento debe ser reconstituido bajo condiciones asépticas.
Fostipur Kit debe ser reconstituido únicamente con el disolvente provisto en el kit.
Prepare una superficie limpia y lave sus manos antes de reconstituir la solución. Es importante que tanto sus manos como los utensilios que vaya a utilizar estén lo más limpios posible.
Extienda los siguientes materiales sobre una superficie limpia:
- 2 trozos de algodón con alcohol (no vienen en la caja),
- 1 vial que contiene el polvo de Fostipur Kit,
- 1 jeringa precargada con disolvente,
- 1 aguja para preparar la inyección,
- 1 aguja fina para la inyección subcutánea.
Reconstitución de la solución para la inyección utilizando 1 vial de polvo.
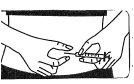
Preparar la solución para la inyección:
|
|
|
|
|
NO AGITE, pero mueva suavementeel vial entre sus manos hasta que el polvo esté completamente disuelto, intentando evitar la creación de espuma. |
|
Preparación de dosis más altas, utilizando más de 1 vial de polvo
Si su médico le ha recomendado dosis más altas, usted lo puede conseguir utilizando más de 1 vial de polvo con una jeringa precargada de disolvente.
Cuando se reconstituye más de 1 vial de Fostipur Kit, al final de la fase 4 descrita anteriormente, introduzca el contenido reconstituido del primer vial otra vez en la jeringa, e inyéctelo lentamente en un segundo vial. Repita las fases 2 a 4 para el segundo vial y posteriores hasta que se disuelva el contenido del número requerido de viales equivalente a la dosis prescrita (dentro del límite de la dosis máxima total de 450 UI, correspondiente a un máximo de 6 viales de Fostipur Kit 75 UI, 3 viales de Fostipur Kit 150 UI, o 2 viales de Fostipur Kit 225 UI).
Su médico puede incrementarle la dosis en 37,5 UI, que representa la mitad de un vial de Fostipur Kit 75 UI.
Para ello, usted debe reconstituir el contenido del vial de 75 UI de acuerdo con las fases 2 a 3 descritas anteriormente e introducir la mitad de esta solución reconstituida (0,5 ml) en la jeringa de acuerdo con la fase 4.
En esa situación, usted tendrá dos preparaciones a inyectar: la primera preparación reconstituida en 1 ml y la segunda conteniendo 37,5 UI en 0,5 ml.
Ambas preparaciones deberán ser inyectadas con sus propias jeringas de acuerdo con las siguientes fases.
La solución debe ser transparente e incolora.
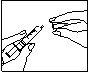
Inyecte el medicamento por vía subcutánea:
|
|
|
|
El sitio de la inyección:
- Su médico o enfermera le habrán indicado en qué parte de su cuerpo puede inyectarse el medicamento.
Los lugaresmás comunes sonel muslo ola pared abdominal inferiorpor debajo del ombligo.
- Limpie el sitio de la inyección con un algodón con alcohol.
Colocación de la aguja:
|
|
Inyección de la solución:
- Inyéctesela bajo la piel tal como le enseñaron. No se la inyecte directamente en una vena. Empuje el émbolo lentamente y de forma constante, de modo que la solución sea inyectada correctamente y la piel no se dañe.
Tómese todo el tiempoque necesitepara inyectarse el volumen de la soluciónprescrita.Tal como se describe en la preparación de la solución, dependiendo de la dosis prescrita por su médico, puede que no tenga que utilizar el volumen total de la solución.
Extracción de la aguja:
- Saque la jeringa rápidamente y presione en el sitio de la inyección con un algodón con desinfectante. Un suave masaje en el lugar, mientras que todavía mantiene la presión, ayuda a dispersar la solución de Fostipur Kit y alivia las molestias.
Eliminación de todos los utensilios utilizados:
Cualquier producto no utilizado o material de desecho debe eliminarse según los requisitos locales (una vez finalizada la inyección todas las agujas y jeringas vacías deben desecharse en un contenedor apropiado).
Si usa más Fostipur Kit del que debe
Los efectos de una sobredosis de Fostipur Kit son desconocidos, aunque es de suponer que podría ocurrir un síndrome de hiperestimulación ovárica (ver Posibles efectos adversos). Si se administra más Fostipur Kit del que debe, póngase en contacto con su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o al Servicio de Información Toxicológica, teléfono: 915 620 420, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Fostipur Kit
Póngase la próxima inyección, en el momento que estaba previsto. No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Fostipur Kit
No interrumpa el tratamiento bajo su propia iniciativa. Consulte siempre a su médico si usted está considerando dejar de usar este medicamento. Si tiene cualquier otra duda sobre el uso de este medicamento, consulte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Fostipur Kit puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos siguientes son importantes y requerirán actuación inmediata si los experimenta.
Debe interrumpir la administración de Fostipur Kit y visitar a su médico inmediatamente si ocurre lo siguiente:
Frecuentes, pueden afectar a entre 1 y 10 de cada 100 personas:
- Síndrome de Hiperestimulación Ovárica ( ver Sección 2 de la información adicional)
También se ha informado sobre los efectos adversos siguientes:
Frecuentes, pueden afectar a entre 1 y 10 de cada 100 personas:
- Dolor de cabeza,
- sensación de hinchazón en el abdomen,
- estreñimiento,
- dolor en la zona de la inyección.
Poco frecuentes, pueden afectar a entre 1 y 10 de cada 1000 personas:
- Aumento de la actividad de la glándula tiroidea,
- cambios de humor,
- cansancio,
- mareos,
- dificultad para respirar (disnea),
- sangrado por la nariz,
- náuseas, indigestión, dolor abdominal,
- enrojecimiento, picazón,
- sofocos,
- cistitis,
- aumento de las mamas, dolor de mamas,
- dificultad para detener las hemorragias.
Puede producirse enrojecimiento, dolor y hematomas en el lugar de la inyección (frecuencia no establecida).
Ver sección 2 de la información adicional sobre el riesgo de coágulos sanguíneos, embarazo ectópico, embarazos múltiples y abortos.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fostipur Kit
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25ºC. Conservar el vial y la jeringa precargada de disolvente en el embalaje exterior para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja de cartón y en el vial.
Utilizar inmediatamente después de la reconstitución.
No utilice Fostipur Kit si usted advierte que la solución no parece transparente. Tras la reconstitución la solución debe ser transparente e incolora.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fostipur Kit
El principio activo es urofolitropina.
Cada vial contiene 300 UI de urofolitropina (hormona foliculostimulante FSH): 1 ml de solución reconstituida contiene 300 UI de urofolitropina.
La actividad específica in vivoes igual o superior a 5000 UI de FSH por mg de proteína.
Los demás componentes son:
Polvo: lactosa monohidrato.
Disolvente: cloruro de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Fostipur Kit se presenta en polvo y disolvente para solución inyectable.
Cajas con 1, 5 ó 10 kits. Cada kit contiene: 1 vial con polvo que contiene 300 UI de urofolitropina, 1 jeringa precargada con 1 ml de disolvente, 1 aguja para la reconstitución y 1 aguja para la inyección subcutánea.
El aspecto del polvo es de una masa endurecida blanca a blanquecina, y el disolvente es transparente e incoloro.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia 2
26900 Lodi (Italia)
Responsable de la fabricación
IBSA Farmaceutici Italia Srl., Via Martiri di Cefalonia, 2 - 26900 Lodi (Italia)
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (España)
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres (las concentraciones y formas farmacéuticas son idénticas en todos los países, sólo cambian los nombres comerciales)
Austria: Fostimon PFS
Bélgica: Fostimon
Chipre: Fostimon PFS
Dinamarca: Fostimon Set
Finlandia: Fostimon Set
Francia: Fostimonkit
Luxemburgo: Fostimon
Irlanda: Fostimon PFS
Holanda: Fostimon Set
Noruega. Fostimon Set
España: Fostipur Kit
Suecia: Fostimon Set
Gran Bretaña: Fostimon PFS
Fecha de la última revisión de este prospecto: febrero 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FOSTIPUR KIT 300 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 150 UI urofolitropina/ mlPrincipio activo: UrofolitropinaFabricante: Ibsa Farmaceutici Italia S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, 75 IU para 1 ml de solución/5000 iu de FSH por mgPrincipio activo: UrofolitropinaFabricante: Ibsa Farmaceutici Italia S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, 150 UI PARA 1 ML DE SOLUCIÓN RECONSTITUIDAPrincipio activo: UrofolitropinaFabricante: Ibsa Farmaceutici Italia S.R.L.Requiere receta
Médicos online para FOSTIPUR KIT 300 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FOSTIPUR KIT 300 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





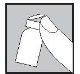

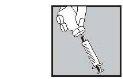 Con la aguja aún insertada, dé la vuelta al vial hacia abajo.
Con la aguja aún insertada, dé la vuelta al vial hacia abajo.