ZYCLARA 3,75% CREMA

Cómo usar ZYCLARA 3,75% CREMA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Zyclara 3,75% crema
imiquimod
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Zyclara y para qué se utiliza
- Qué necesita saber antes de empezar a usar Zyclara
- Cómo usar Zyclara
- Posibles efectos adversos
- Conservación de Zyclara
- Contenido del envase e información adicional
1. Qué es Zyclara y para qué se utiliza
Zyclara 3,75% crema contiene la sustancia activa imiquimod.
Este medicamento se prescribe para el tratamiento de la queratosis actínica en adultos, que es un Modificador de la Respuesta Inmune (para estimular el sistema inmunológico humano).
Este medicamento estimula su propio sistema inmunitario para producir sustancias naturales que contribuyen a combatir su queratosis actínica.
Las queratosis actínicas aparecen como zonas de piel rugosa presentes en personas que han estado expuestas a gran cantidad de luz solar en el transcurso de su vida. Estas zonas pueden ser del mismo color que su piel, o ser grisáceas, rosadas, rojas o marrones. Pueden ser planas y escamosas, o elevadas, rugosas, duras y verrugosas.
Este medicamento se debe usar exclusivamente en queratosis actínicas del rostro o del cuero cabelludo, si su médico ha decidido que es el tratamiento más adecuado para usted.
2. Qué necesita saber antes de empezar a usar Zyclara
No use Zyclara
- si es alérgico al imiquimod o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar usar Zyclara:
- si ha utilizado anteriormente este medicamento u otro preparado similar, en una concentración diferente.
- si padece trastornos autoinmunes.
- si ha tenido un transplante de órgano.
- si tiene recuento sanguíneo anormal.
Instrucciones generales durante el tratamiento
- Si se ha sometido recientemente a tratamiento quirúrgico o farmacológico, espere hasta que la zona a tratar haya cicatrizado antes de usar este medicamento.
- Evite el contacto con ojos, labios y fosas nasales. En caso de contacto accidental, elimine la crema lavando la zona con agua.
- Utilice la crema sólo de forma externa (sobre la piel del rostro o cuero cabelludo).
- No use una cantidad de crema mayor que la recomendada por su médico.
- No cubra la zona tratada con vendajes u otros elementos después de haber aplicado Zyclara.
- Si desarrolla molestias excesivas en la zona tratada, retire la crema con un jabón neutro y agua. Una vez que hayan remitido las molestias, puede reanudar el tratamiento de la forma recomendada. La crema no se debe aplicar más de una vez al día.
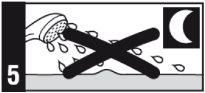
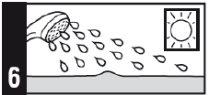
- No utilice lámparas solares ni soláriums, y evite en la mayor medida posible la luz del sol durante el tratamiento con este medicamento. Si sale al aire libre durante el día, utilice pantalla solar y vista prendas protectoras, así como un sombrero de ala ancha.
Reacciones cutáneas locales
Mientras utilice Zyclara puede experimentar reacciones cutáneas locales debido a la forma en que el medicamento actúa sobre su piel. Estas reacciones pueden indicar que el medicamento está actuando del modo previsto.
Durante el uso de Zyclara y hasta la curación, la zona de tratamiento puede presentar un aspecto claramente diferente al de la piel normal. Existe también la posibilidad de que una inflamación pre-existente empeore temporalmente.
Este medicamento también puede provocar síntomas similares a los de la gripe (incluidos cansancio, náuseas, fiebre, dolores musculares y articulares, y escalofríos) antes o durante la aparición de las reacciones cutáneas locales.
Si se presentan síntomas gripales o sensación de malestar o reacciones cutáneas locales intensas, puede tomarse un período de descanso de varios días. Usted podría reanudar el tratamiento con imiquimod crema después de que la reacción cutánea se haya moderado. Sin embargo, no debe prolongarse el ciclo de tratamiento de 2 semanas debido a las dosis pérdidas o a los períodos de descanso.
La intensidad de las reacciones cutáneas locales tiende a ser más baja en el segundo ciclo que en el primer ciclo de tratamiento con Zyclara.
La respuesta al tratamiento no se puede evaluar adecuadamente hasta la resolución de las reacciones cutáneas locales. Deberá continuar el tratamiento de la forma prescrita.
Este medicamento puede poner de manifiesto y tratar queratosis actínicas que no hayan sido vistas o percibidas previamente, y que posteriormente pueden desaparecer. La aplicación deberá continuar durante la totalidad del ciclo de tratamiento aun cuando todas las queratosis actínicas parezcan haber desaparecido.
Niños y adolescentes
Este medicamento no se debe administrar a niños menores de 18 años, porque no se han establecido la seguridad y eficacia en pacientes menores de dicha edad. No se dispone de datos acerca del uso de imiquimod en niños y adolescentes.
Otros medicamentos y Zyclara
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Informe a su médico antes de iniciar el tratamiento si usted recibe medicamentos inmunosupresores que inhiben el sistema inmune.
Evite el uso concomitante de Zyclara y cualquier otra crema de imiquimod en el mismo área de tratamiento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Su médico valorará los riesgos y los beneficios de utilizar Zyclara durante el embarazo. Los estudios en animales no indican efectos nocivos directos o indirectos en el embarazo.
Se desconoce si imiquimod pasa a la leche materna. No debe usar Zyclara si está dando el pecho o tiene previsto dar el pecho. Su médico decidirá si debe interrumpir la lactancia o si debe interrumpir el tratamiento con Zyclara
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y usar máquinas es nula o insignificante.
Zyclara contiene parahidroxibenzoato de metilo, parahidroxibenzoato de propilo, alcohol cetílico, alcohol estearílico y alcohol bencílico.
Puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo (E 218) y parahidroxibenzoato de propilo (E 216).
Este medicamento puede producir reacciones locales en la piel (como dermatitis de contacto) porque contiene alcohol cetílico y alcohol estearílico.
Este medicamento contiene 5 mg de alcohol bencílico en cada sobre. El alcohol bencílico puede provocar reacciones alérgicas e irritación local moderada.
3. Cómo usar Zyclara
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. No utilice este medicamento hasta que su médico le haya enseñado la manera correcta de hacerlo.
Este medicamento sólo se debe usar para las queratosis actínicas del rostro y cuero cabelludo.
Dosificación
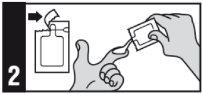
Aplique este medicamento sobre la zona afectada una vez al día, inmediatamente antes de acostarse.
La dosis diaria máxima es de 2 sobres (500 mg = 2 sobres de 250 mg cada uno).
Este medicamento no se debe aplicar sobre zonas de tamaño mayor que todo el rostro o el cuero cabelludo alopécico.
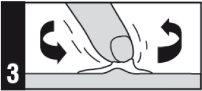
Forma de administración
|
|
|
|
|
|
|
|
|
|
|
|
Duración del tratamiento
El tratamiento se inicia con una aplicación diaria durante dos semanas, seguido de una pausa sin aplicación durante dos semanas, y finaliza con una aplicación diaria durante dos semanas nuevamente.
Si usa más Zyclara del que debe
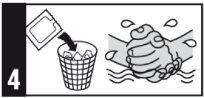
Si se ha aplicado demasiada crema, retire el exceso lavando la zona con agua y jabón neutro.
Cuando haya remitido cualquier reacción cutánea, puede reanudar el tratamiento según el programa regular recomendado. No se debe aplicar la crema más de una vez al día.
Si traga accidentalmente este medicamento, póngase de inmediato en contacto con su médico.
Si olvidó usar Zyclara
Si olvidó una dosis de Zyclara, espere hasta la noche siguiente para aplicarla y, a continuación, prosiga con el régimen habitual. La crema no se debe aplicar más de una vez al día. Cada ciclo de tratamiento no deberá durar más de dos semanas, incluso si ha omitido algunas dosis.
Si interrumpe el tratamiento con Zyclara
Consulte con su médico antes de suspender el tratamiento con Zyclara.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Solicite atención médica inmediata si aparece cualquiera de estos efectos adversos graves durante el uso de este medicamento:
Reacciones cutáneas graves (frecuencia no conocida), con lesiones cutáneas o manchas en la piel que se inician como pequeñas zonas de color rojo y evolucionan hasta alcanzar el aspecto de pequeñas dianas, posiblemente con síntomas tales como picor, fiebre, malestar general, dolores articulares, problemas de visión, sensación de quemadura, dolores o picores oculares y llagas bucales. Si sufre estos trastornos, interrumpa el uso de este medicamento e informe inmediatamente a su médico.
En algunas personas se ha observado un descenso de la fórmula sanguínea (frecuencia no conocida). Este hecho podría aumentar su susceptibilidad a sufrir infecciones, desarrollar hematomas o provocar cansancio. Si percibe cualquiera de estos síntomas, informe a su médico.
Algunos pacientes que padecen transtornos autoinmunes pueden experimentar empeoramiento de su enfermedad. Consulte a su médico, si experimenta cualquier cambio durante el tratamiento con Zyclara.
Si hay presencia de pus u otro signo de infección en la piel (frecuencia no conocida), consúltelo con su médico.
Muchos de los efectos secundarios de este medicamento se deben a su acción local sobre la piel. Las reacciones cutáneas locales pueden indicar que el medicamento está actuando de la forma prevista. Si su piel reacciona mal o sufre molestias excesivas durante el uso de este medicamento, suspenda la aplicación de la crema y lave la zona con agua y un jabón neutro. Seguidamente, póngase en contacto con su médico o farmacéutico, quien puede aconsejarle que interrumpa la aplicación de este medicamento durante algunos días (es decir, realizar un breve descanso durante el tratamiento).
Se han comunicado los siguientes efectos adversos con imiquimod:
Muy frecuentes(puede afectar a más de 1 de cada 10 personas)
- Enrojecimiento de la piel, costras, descamación cutánea, supuración, sequedad de la piel, hinchazón cutánea, úlceras cutáneas y reducción de la pigmentación cutánea en el sitio de aplicación.
Frecuentes(puede afectar hasta 1 de cada 10 personas)
- Otras reacciones adicionales en el sitio de aplicación, por ej., inflamación de la piel, prurito, dolor, sensación de quemadura, irritación y exantema cutáneo
- Glándulas inflamadas
- Cefaleas
- Vértigo
- Pérdida de apetito
- Náuseas
- Diarrea
- Vómitos
- Síntomas semejantes a la gripe
- Fiebre
- Dolor
- Dolores musculares y articulares
- Dolor torácico
- Insomnio
- Cansancio
- Infección vírica (herpes simple)
- Aumento de la glucosa en sangre
Poco frecuentes(puede afectar hasta 1 de cada 100 personas)
- Alteraciones en la zona de aplicación, por ej., sangrado, pequeñas zonas hinchadas en la piel, inflamación, hormigueo, aumento de la sensibilidad al contacto, formación de cicatrices, sensación de calor, lesiones de la piel, ampollas o pústulas
- Debilidad
- Escalofríos
- Falta de energía (letargo)
- Molestias
- Tumefacción del rostro
- Lumbalgia
- Dolor de extremidades
- Nariz taponada
- Dolor de garganta
- Irritación de los ojos
- Hinchazón de los párpados
- Depresión
- Irritabilidad
- Boca seca
- Dolor abdominal
Raros(puede afectar hasta 1 de cada 1.000 personas)
- Brotes de enfermedades autoinmunes (una enfermedad autoinmune es una enfermedad causada por una respuesta inmunitaria anormal)
- Reacciones cutáneas alejadas del sitio de aplicación
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- Cambios de color de la piel
Algunos pacientes han experimentado cambios de color de la piel en la zona sobre la que se aplicó Zyclara. Aunque estos cambios tienden a mejorar con el tiempo, en algunos pacientes pueden ser permanentes
- Pérdida de pelo
Un número reducido de pacientes ha experimentado pérdida de pelo en la zona de tratamiento o inmediatamente adyacente
- Elevación de las enzimas hepáticas
Ha habido informes de un incremento de las enzimas hepáticas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
.
5. Conservación de Zyclara
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase exterior después de CAD.
La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25°C.
Una vez abiertos, los sobres no se deben reutilizar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Zyclara
- El principio activo es imiquimod. Cada sobre contiene 9,375 mg de imiquimod en 250 mg de crema (100 mg de crema contienen 3,75 mg de imiquimod).
- Los demás componentes son: ácido isoesteárico, alcohol bencílico, alcohol cetílico, alcohol estearílico, parafina blanca ligera, polisorbato 60, estearato de sorbitan,glicerol,parahidroxibenzoato de metilo (E 218),parahidroxibenzoato de propilo (E 216),goma xantán, agua purificada (ver también la sección 2 “Zyclara contiene parahidroxibenzoato de metilo, parahidroxibenzoato de propilo, alcohol cetílico, alcohol estearílico y alcohol bencílico”).
Aspecto de Zyclara y contenido del envase
- Cada sobre de Zyclara 3,75% crema contiene 250 mg de una crema de color blanco a ligeramente amarillenta, de aspecto uniforme.
- Cada envase contiene 14, 28 ó 56 sobres de un solo uso de poliéster/ polietileno de baja densidad blanco/lámina de aluminio.
Puede que sólo estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart
Dublin 15
DUBLIN
Irlanda
Responsable de la fabricación
Swiss Caps GmbH
Grassingerstraße 9
83043 Bad Aibling
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tél/Tel: +32 2 658 61 00 | Luxembourg/Luxemburg Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tél/Tel: +32 2 658 61 00 |
???????? ?????? ???? ???.: +359 2 44 55 400 | Magyarország Mylan EPD Kft. 1138 Budapest Váci út 150 Tel: +36 1 465 2100 |
Ceská republika Viatris CZ s.r.o. Tel: +420 222 004 400 | Malta V.J. Salomone Pharma Limited Upper Cross Road Marsa, MRS 1542 Tel: +356 21 22 01 74 |
Danmark Viatris ApS Borupvang 1 2750 Ballerup Tlf: +45 28 11 69 32 | Nederland Mylan Healthcare B.V. Krijgsman 20 1186 DM Amstelveen Tel: +31 (0)20 426 3300 |
Deutschland Viatris Healthcare GmbH Lütticher Straße 5 53842 Troisdorf Tel: +49 800 0700 800 | Norge Viatris AS Hagaløkkveien 26 1383 Asker Tlf: +47 66 75 33 00 |
Eesti Meda Pharma SIA Liivalaia 13/15 11018 Tallinn Tel: +372 62 61 025 | Österreich Mylan Österreich GmbH Guglgasse 15 1110 Wien Tel: + 43 (0)1 86 390 0 |
Ελλ?δα Viatris Hellas Ltd Τηλ: +30 210 010 0002 | Polska Mylan Healthcare Sp. z.o.o. ul. Postepu 21B 02-676 Warszawa Tel: +48 22 546 6400 |
España Viatris Pharmaceuticals, S.L.U. Tel: +34 900 102 712 | Portugal Viatris Healthcare, Lda. Av. D. João II, Edifício Atlantis, nº 44C – 7.3 e 7.4 1990-095 Lisboa Tel: +351 214 127 200 |
France Viatris Médical 1 bis place de la Défense – Tour Trinity 92400 Courbevoie Tél: +33 (0)1 40 80 15 55 | România BGP PRODUCTS SRL Tel.: +40 372 579 000 |
Hrvatska Viatris Hrvatska d.o.o. Koranska 2 10 000 Zagreb Tel: +385 1 2350 599 | Slovenija Viatris d.o.o. Tel: +386 1 23 63 180 |
Ireland Mylan Ireland Limited Tel: +353 1 8711600 | Slovenská republika Viatris Slovakia s.r.o. Tel: +421 2 32 199 100 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Viatris Oy Vaisalantie 2-8/Vaisalavägen 2-8 02130 Espoo/Esbo Puh/Tel: +358 20 720 9555 |
Italia Mylan Italia Via Vittor Pisani, 20 20124 Milano Tel: +39 0261246921 | Sverige Viatris AB Box 23033 104 35 Stockholm +46 (0) 8 630 19 00 |
Κ?προς Βαρν?βας Χατζηπαναγ?ς Λτδ Λεωφ. Γι?ννου Κρανιδι?τη 226 ΤK 2234, Λατσι?, Λευκωσ?α Τηλ.: +357 22207700 | United Kingdom (Northern Ireland) Mylan IRE Healthcare Limited Tel: +353 18711600 |
Latvija Meda Pharma SIA 101 Mukusalas str. Riga LV-1004 Talr: +371 67616137 | |
Lietuva Meda Pharma SIA Žalgirio str. 90-100 Vilnius LT-09303 Tel. + 370 52051288 |
Fecha de la última revisión de este prospecto: (MM/AAAA)
La información detallada de este medicamento está disponible en la página Web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Precio medio en farmacia66.11 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZYCLARA 3,75% CREMAForma farmacéutica: CREMA, 50 mg/gPrincipio activo: ImiquimodFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: CREMA, 50 mg/gPrincipio activo: ImiquimodFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: CREMA, 5 %Principio activo: ImiquimodFabricante: Mibe Pharma Espana S.L.Requiere receta
Médicos online para ZYCLARA 3,75% CREMA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZYCLARA 3,75% CREMA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes