
ZALASTA 20 MG COMPRIMIDOS BUCODISPERSABLES EFG


Cómo usar ZALASTA 20 MG COMPRIMIDOS BUCODISPERSABLES EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Zalasta 5 mg comprimidos bucodispersables EFG
Zalasta 7,5 mg comprimidos bucodispersables EFG
Zalasta 10 mg comprimidos bucodispersables EFG
Zalasta 15 mg comprimidos bucodispersables EFG
Zalasta 20 mg comprimidos bucodispersables EFG
Olanzapina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Zalasta y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Zalasta
- Cómo tomar Zalasta
- Posibles efectos adversos
- Conservación de Zalasta
- Contenido del envase e información adicional
1. Qué es Zalasta y para qué se utiliza
Zalasta contiene el principio activo olanzapina. Zalasta pertenece al grupo de los medicamentos denominados los antipsicóticos para tratar las siguientes enfermedades:
- Esquizofrenia, una enfermedad cuyos síntomas son oír, ver o sentir cosas irreales, creencias erróneas, suspicacia inusual, y volverse retraído. Las personas que sufren estas enfermedades pueden encontrarse, además, deprimidas, con ansiedad o tensas.
- Trastorno maníaco de moderado a grave, caracterizado por síntomas tales como excitación o euforia.
Zalasta ha demostrado prevenir la recurrencia de estos síntomas en pacientes con trastorno bipolar cuyos episodios maníacos han respondido al tratamiento con olanzapina.
2. Qué necesita saber antes de empezar a tomar Zalasta
No tome Zalasta
- Si es alérgico a la olanzapina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6). La reacción alérgica puede manifestarse en forma de erupción, picor, hinchazón de la cara o de los labios o dificultad para respirar. Si le pasara esto, dígaselo a su médico.
- Si previamente se le ha diagnosticado problemas en los ojos tales como ciertos tipos de glaucoma (aumento de la presión en el ojo).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Zalasta
- No se recomienda el uso de Zalasta en pacientes de edad avanzada con demencia ya que puede tener efectos adversos graves.
- Medicamentos de este tipo pueden provocar movimientos inusuales, sobre todo en la cara o en la lengua. Si le pasara esto después de haber tomado Zalasta, dígaselo a su médico.
- Muy raramente, medicamentos de este tipo producen una combinación de fiebre, respiración acelerada, sudoración, rigidez muscular y un estado de obnubilación o somnolencia. Si le ocurriera esto, póngase en contacto con su médico inmediatamente.
- Se ha observado un aumento de peso en los pacientes que están tomando Zalasta. Usted y su médico deben comprobar su peso con regularidad. Si fuera necesario, su médico le puede ayudar a planificar una dieta o considerar la posibilidad de remitirle a un nutricionista.
- Se han observado niveles elevados de azúcar y grasas (triglicéridos y colesterol) en sangre en los pacientes que están tomando Zalasta. Su médico debe hacerle análisis de sangre para controlar su azúcar en sangre y los niveles de grasa antes de que comience a tomar Zalasta y de forma regular durante el tratamiento.
- Si usted o alguien en su familia tiene antecedentes de coágulos sanguíneos, consulte con su médico, ya que los medicamentos de este tipo han sido asociados con la formación de coágulos en la sangre.
Si usted padece cualquiera de las siguientes enfermedades, hágaselo saber a su médico lo antes posible:
- Infarto cerebral o falta de riego sanguíneo transitorio en el cerebro (síntomas pasajeros de infarto cerebral).
- Enfermedad de Parkinson
- Problemas de próstata
- Bloqueo intestinal (Íleo paralítico)
- Enfermedad del hígado o riñón
- Alteraciones de la sangre
- Enfermedades del corazón
- Diabetes
- Convulsiones
- Si cree que puede tener pérdida de sales como consecuencia de tener diarrea y vómitos intensos de forma prolongada o por el uso de medicamentos diuréticos (comprimidos para orinar)
Si sufre demencia, usted o su cuidador o familiar deben informar a su médico si ha tenido alguna vez un infarto cerebral o una falta de riego sanguíneo en el cerebro.
Como precaución rutinaria, si tiene más de 65 años, convendría que su médico le controlara la tensión arterial.
Niños y adolescentes
Los pacientes menores de 18 años no deben tomar Zalasta.
Uso de Zalasta con otros medicamentos
Sólo use otros medicamentos al mismo tiempo que Zalasta, si su médico se lo autoriza. Es posible que sienta cierta sensación de sueño si combina Zalasta con antidepresivos o medicamentos para la ansiedad o que ayuden a dormir (tranquilizantes).
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
- En concreto, diga a su médico si está tomando: medicación para la enfermedad de Parkinson
- carbamazepina (un antiepiléptico y estabilizador del humor), fluvoxamina (un antidepresivo) o ciprofloxacino (un antibiótico). Puede que necesiten cambiar su dosis de Zalasta.
Uso de Zalasta con alcohol
No debe beber alcohol si le han administrado Zalasta porque puede producir somnolencia.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. No debe tomar este medicamento cuando esté dando el pecho ya que pequeñas cantidades de Zalasta pueden pasar a la leche materna.
Los siguientes síntomas pueden ocurrir en recién nacidos, de madres que han usado Zalasta en el último trimestre (últimos tres meses de su embarazo): temblores, rigidez muscular y/o debilidad, somnolencia, agitación, problemas respiratorios y dificultad para comer. Si su bebé tiene cualquiera de estos síntomas, póngase en contacto con su médico.
Conducción y uso de máquinas
Existe el riesgo de sufrir somnolencia cuando esté tomando Zalasta. Si le ocurriera esto, no conduzca vehículos ni use maquinaria. Consúltelo con su médico.
Zalasta contiene aspartamo
Este medicamento contiene 0,50 mg de aspartamo en cada comprimido bucodispersable de 5 mg.
Este medicamento contiene 0,75 mg de aspartamo en cada comprimido bucodispersable de 7,5 mg.
Este medicamento contiene 1,00 mg de aspartamo en cada comprimido bucodispersable de 10 mg.
Este medicamento contiene 1,50 mg de aspartamo en cada comprimido bucodispersable de 15 mg.
Este medicamento contiene 2,00 mg de aspartamo en cada comprimido bucodispersable de 20 mg.
Aspartamo contiene una fuente de fenilalanina que puede ser perjudicial en caso de padecer (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
3. Cómo tomar Zalasta
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico le indicará cuántos comprimidos de Zalasta debe tomar y durante cuánto tiempo. La dosis diaria de Zalasta oscila entre 5 mg y 20 mg.
Consulte con su médico si vuelve a sufrir los síntomas pero no deje de tomar Zalasta a menos que se lo diga su médico.
Los comprimidos de Zalasta se deben tomar una vez al día, siguiendo las indicaciones de su médico.
Procure tomar los comprimidos a la misma hora todos los días. Puede tomarlos con o sin alimentos.
Cómo tomar Zalasta
Zalasta comprimidos se desmoronan fácilmente por lo que se deben manipular con cuidado. No manipule los comprimidos con las manos húmedas porque se pueden deshacer. Extraiga el comprimido del envoltorio como sigue:
- Sujete el blíster por los lados y separe un envoltorio individual del resto del blíster a lo largo de las perforaciones.
- Tire del borde del aluminio y extráigalo completamente.
- Deje caer el comprimido en la mano.
- Deposite el comprimido en la lengua inmediatamente.
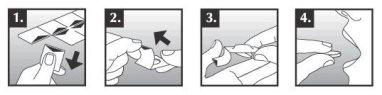
En unos segundos, el comprimido comienza a desintegrarse y puede ser tragado con o sin agua. La boca debe estar vacía antes de depositar éste en la lengua.
También se puede echar el comprimido en una taza o en un vaso lleno de agua. Se debe beber inmediatamente.
Si toma más Zalasta del que debe
Los pacientes que han tomado más Zalasta del que debían, han experimentado los siguientes síntomas: latidos rápidos del corazón, agitación/agresividad, problemas con el habla, movimientos inusuales (especialmente de la cara y de la lengua) y un nivel reducido de consciencia. Otros síntomas pueden ser: confusión aguda, convulsiones (epilepsia), coma, una combinación de fiebre, respiración rápida, sudor, rigidez muscular, somnolencia o letargo, enlentecimiento de la frecuencia respiratoria, aspiración, aumento de la tensión arterial o disminución de la tensión arterial, ritmos anormales del corazón. Póngase en contacto con su médico o diríjase inmediatamente al hospital si nota cualquiera de los síntomas antes mencionados. Enséñele al médico el envase con los comprimidos.
Si olvidó tomar Zalasta
Tome sus comprimidos tan pronto como se acuerde. No tome una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Zalasta
No interrumpa el tratamiento simplemente porque note que se encuentra mejor. Es muy importante que continúe tomando Zalasta mientras se lo diga su médico.
Si deja de tomar Zalasta de forma repentina, pueden aparecer síntomas como sudoración, imposibilidad para dormir, temblor, ansiedad, o náuseas y vómitos. Su médico puede sugerirle que reduzca la dosis gradualmente antes de dejar el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede tener efectos adversos, aunque no todas las personas los sufran.
Póngase en contacto inmediatamente con su médico si usted tiene:
- movimientos inusuales (un efecto adverso frecuente que puede afectar hasta 1 de cada 10 personas) especialmente de la cara o de la lengua.
- coágulos sanguíneos en las venas (un efecto adverso poco frecuente que puede afectar hasta 1 de cada 100 personas), especialmente en las piernas (los síntomas incluyen sudoración, dolor y enrojecimiento en la pierna), que pueden viajar a través de la sangre hacia los pulmones, causando dolor en el pecho y dificultad para respirar. Si experimenta cualquiera de estos síntomas, acuda al médico de inmediato.
- combinación de fiebre, respiración acelerada, sudoración, rigidez muscular y un estado de obnubilación o somnolencia (la frecuencia no puede ser estimada a partir de los datos disponibles)
Efectos adversos muy frecuentes (que pueden afectar a más de 1 entre 10 personas) incluyen aumento de peso, somnolencia, y aumento de los niveles de prolactina en sangre. En las primeras fases del tratamiento, algunas personas pueden sentir mareos o desmayos (con latidos del corazón más lentos), sobre todo al incorporarse cuando están tumbados o sentados. Esta sensación suele desaparecer espontáneamente, pero si no ocurriera así, consulte con su médico.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas) incluyen cambios en los niveles de algunas células sanguíneas, lípidos circulantes y al comienzo del tratamiento aumentos temporales de las enzimas hepáticas, aumento de los niveles de azúcar en sangre y orina, aumento de los niveles de ácido úrico y creatinfosfoquinasa en sangre, aumento del apetito, mareos, agitación, temblor, movimientos extraños (discinesia), estreñimiento, sequedad de boca, erupción en la piel, pérdida de fuerza, cansancio excesivo, retención de líquidos que provoca inflamación de las manos, los tobillos o los pies, fiebre, dolor en las articulaciones y disfunciones sexuales tales como disminución de la libido en hombres y mujeres o disfunción eréctil en hombres.
Efectos adversos poco frecuentes (pueden afectar hasta 1 persona de cada 100) incluyen hipersensibilidad (p. ej. inflamación de la boca y de la garganta, picores, erupción en la piel), diabetes o empeoramiento de la diabetes, relacionados ocasionalmente con cetoacidosis (acetona en sangre y orina) o coma, convulsiones, en la mayoría de los casos se relacionan con antecedentes de convulsiones (epilepsia), rigidez muscular o espasmos (incluyendo movimientos de los ojos), síndrome de piernas inquietas, problemas con el habla, tartamudeo; pulso lento, sensibilidad a la luz del sol, sangrado por la nariz, distensión abdominal, pérdida de memoria u olvidos, incontinencia urinaria, pérdida de la habilidad para orinar, pérdida de cabello, ausencia o disminución de los periodos menstruales y cambios en la glándula mamaria en hombres y en mujeres tales como producción anormal de leche materna o crecimiento anormal.
Efectos adversos raros (pueden afectar hasta 1 persona de cada 1000) incluyen descenso de la temperatura corporal normal, ritmo anormal del corazón, muerte repentina sin explicación aparente, inflamación del páncreas, que provoca fuerte dolor de estómago, fiebre y malestar, enfermedad del hígado, con aparición de coloración amarillenta en la piel y en las zonas blancas del ojo, trastorno muscular que se presenta como dolores sin explicación aparente y erección prolongada y/o dolorosa.
Se han comunicado reacciones alérgicas graves, como el Síndrome de Reacción a Fármaco con Eosinofilia y Síntomas Sistémicos (DRESS) DRESS se manifiesta inicialmente con síntomas similares a los de la gripe, con erupción cutánea en la cara que se extiende luego a otras zonas, fiebre, hinchazón de los ganglios linfáticos, niveles elevados de enzimas hepáticas en los análisis de sangre y aumento de un tipo de glóbulos blancos (eosinofilia).
Durante el tratamiento con olanzapina, los pacientes de edad avanzada con demencia pueden sufrir ictus, neumonía, incontinencia urinaria, caídas, cansancio extremo, alucinaciones visuales, una subida de la temperatura corporal, enrojecimiento de la piel y tener problemas al caminar. Se han notificado algunos fallecimientos en este grupo particular de pacientes.
Zalasta puede empeorar los síntomas en pacientes con enfermedad de Parkinson.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Anexo V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Zalasta
Mantener este medicamento fuera de la vista y del alcance de los niños.
No use este medicamento después de la fecha de caducidad que aparece en el cartón. La fecha de caducidad es el último día del mes que se indica.
Conservar en el embalaje original para protegerlo de la luz y la humedad. Este medicamento no requiere ninguna temperatura especial de conservación.
Los medicamentos no se deben tirar a las aguas residuales o a la basura. Pregunte a su farmacéutico como deshacerse de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Qué contiene Zalasta
- El principio activo es olanzapina. Cada comprimido bucodispersable de Zalasta contiene 5 mg, 7,5 mg, 10 mg, 15 mg o 20 mg de olanzapina.
- Los demás componentes son: manitol, celulosa microcristalina, crospovidona, hidroxipropilcelulosa con bajo grado de sustitución, aspartamo, silicato de calcio, estearato de magnesio.
Ver sección 2 “Zalasta contiene aspartamo”.
Aspecto de Zalasta y tamaño del envase
Los comprimidos bucodispersables de Zalasta 5 mg, 7,5 mg, 10 mg, 15 mg y 20 mg son: amarillos jaspeados, redondos, ligeramente biconvexos, con posibles manchas.
Zalasta 5 mg comprimidos bucodispersables están disponibles en cajas de 14, 28, 35, 56 y 70 comprimidos en blísters.
Zalasta 7,5 mg comprimidos bucodispersables están disponibles en cajas de 14, 28, 35, 56 y 70 comprimidos en blísters.
Zalasta 10 mg comprimidos bucodispersables están disponibles en cajas de 14, 28, 35, 56 y 70 comprimidos en blísters.
Zalasta 15 mg comprimidos bucodispersables están disponibles en cajas de 14, 28, 35, 56 y 70 comprimidos en blísters.
Zalasta 20 mg comprimidos bucodispersables están disponibles en cajas de 14, 28, 35, 56 y 70 comprimidos en blísters.
Titular de la autorización de comercialización
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Eslovenia
Responsable de la fabricación
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Eslovenia KRKA-POLSKA Sp. z o.o., ul. Równolegla 5, 02-235 Warszawa, Polonia TAD Pharma GmbH, Heinz-Lohmann-Straße 5, 27472 Cuxhaven, Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien KRKA Belgium, SA. Tél/Tel: + 32 (0) 487 50 73 62 | Lietuva UAB KRKA Lietuva Tel: + 370 5 236 27 40 |
???????? ???? ???????? ???? Te?.: + 359 (02) 962 34 50 | Luxembourg/Luxemburg KRKA Belgium, SA. Tél/Tel: + 32 (0) 487 50 73 62 (BE) |
Ceská republika KRKA CR, s.r.o. Tel: + 420 (0) 221 115 150 | Magyarország KRKA Magyarország Kereskedelmi Kft. Tel.: + 36 (1) 355 8490 |
Danmark KRKA Sverige AB Tlf: + 46 (0)8 643 67 66 (SE) | Malta
Tel: + 356 21 445 885 |
Deutschland TAD Pharma GmbH Tel: + 49 (0) 4721 606-0 | Nederland KRKA Belgium, SA. Tel: + 32 (0) 487 50 73 62 (BE) |
Eesti KRKA, d.d., Novo mesto Eesti filiaal Tel: + 372 (0) 6 671 658 | Norge KRKA Sverige AB Tlf: + 46 (0)8 643 67 66 (SE) |
Ελλ?δα QUALIA PHARMA S.A. Τηλ: + 30 210 6256177 | Österreich KRKA Pharma GmbH, Wien Tel: + 43 (0)1 66 24 300 |
España KRKA Farmacéutica, S.L. Tel: + 34 911 61 03 81 | Polska KRKA-POLSKA Sp. z o.o. Tel.: + 48 (0)22 573 7500 |
France KRKA France Eurl Tél: + 33 (0)1 57 40 82 25 | Portugal KRKA Farmacêutica, Sociedade Unipessoal Lda. Tel: + 351 (0)21 46 43 650 |
Hrvatska KRKA - FARMA d.o.o. Tel: + 385 1 6312 100 | România KRKA Romania S.R.L., Bucharest Tel: + 4 021 310 66 05 |
Ireland KRKA Pharma Dublin, Ltd. Tel: + 353 1 293 91 80 | Slovenija KRKA, d.d., Novo mesto Tel: + 386 (0) 1 47 51 100 |
Ísland LYFIS ehf. Sími: + 354 534 3500 | Slovenská republika KRKA Slovensko, s.r.o. Tel: + 421 (0) 2 571 04 501 |
Italia KRKA Farmaceutici Milano S.r.l. Tel: + 39 02 3300 8841 | Suomi/Finland KRKA Finland Oy Puh/Tel: + 358 20 754 5330 |
Κ?προς Kipa Pharmacal Ltd. Τηλ: + 357 24 651 882 | Sverige KRKA Sverige AB Tel: + 46 (0)8 643 67 66 (SE) |
Latvija KRKA Latvija SIA Tel: + 371 6 733 86 10 | United Kingdom Consilient Health (UK) Ltd. Tel: + 44(0)203 751 1888 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu/.
- País de registro
- Precio medio en farmacia105.78 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZALASTA 20 MG COMPRIMIDOS BUCODISPERSABLES EFGForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 2,5 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 5 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere receta
Médicos online para ZALASTA 20 MG COMPRIMIDOS BUCODISPERSABLES EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZALASTA 20 MG COMPRIMIDOS BUCODISPERSABLES EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











