XIMLUCI 10 MG/ML SOLUCION INYECTABLE

Cómo usar XIMLUCI 10 MG/ML SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ximluci 10 mg/ml solución inyectable
ranibizumab
ADULTOS
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de que le administren este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ximluci y para qué se utiliza
- Qué necesita saber antes de que le administren Ximluci
- Cómo se administra Ximluci
- Posibles efectos adversos
- Conservación de Ximluci
Contenido del envase e información adicional
1. Qué es Ximluci y para qué se utiliza
Qué es Ximluci
Ximluci es una solución que se inyecta en el ojo. Ximluci pertenece a un grupo de medicamentos denominados agentes antineovascularización. Contiene el principio activo denominado ranibizumab.
Para qué se utiliza Ximluci
Ximluci se utiliza en adultos para tratar varias enfermedades oculares que causan alteración de la visión.
Estas enfermedades son el resultado de una lesión en la retina (capa sensible a la luz en la parte posterior del ojo) provocada por:
- El crecimiento de vasos sanguíneos anómalos, que pierden líquido. Esto se observa en enfermedades como la degeneración macular asociada a la edad (DMAE) y la retinopatía diabética proliferativa (RDP, una enfermedad provocada por la diabetes). También puede ir asociado con la neovascularización coroidea (NVC) debida amiopía patológica (MP), estrías angioides, corioretinopatía serosa central o NVC inflamatoria.
- Edema macular (hinchazón del centro de la retina). La causa de este hinchazón puede ser la diabetes (una enfermedad conocida como edema macular diabético (EMD)) o un bloqueo de las venas retinianas de la retina (una enfermedad conocida como oclusión de la vena de la retina (OVR)).
Cómo actúa Ximluci
Ximluci reconoce y se une de forma específica a una proteína denominada factor de crecimiento endotelial vascular A (VEGF-A) humano presente en los ojos. En exceso, el VEGF-A causa el crecimiento de vasos sanguíneos anómalos e hinchazón en el ojo que puede ocasionar una alteración de la visión en enfermedades como DMAE, EMD, RDP, OVR, MP y NVC. Mediante la unión al VEGF-A, Ximluci puede impedir que actúe y prevenir dicho crecimiento e hinchazón anómalos.
En estas enfermedades, Ximluci puede ayudar a estabilizar y, en muchos casos, mejorar su visión.
2. Qué necesita saber antes de que le administren Ximluci
No le deben administrar Ximluci
- Si es alérgico al ranibizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene una infección en el ojo o alrededor del mismo.
- Si tiene dolor o enrojecimiento (inflamación intraocular grave) en el ojo.
Advertencias y precauciones
Consulte a su médico antes de que le administren Ximluci
- Ximluci se administra mediante una inyección en el ojo. Ocasionalmente, tras el tratamiento con Ximluci puede aparecer una infección en la parte interna del ojo, dolor o enrojecimiento (inflamación), desprendimiento o desgarro de una de las capas situadas en el fondo del ojo (desprendimiento o desgarro de la retina y desprendimiento o desgarro del epitelio pigmentario de la retina), o enturbiamiento del cristalino (catarata). Es importante identificar y tratar tal infección o desprendimiento de retina lo antes posible. Informe inmediatamente a su médico si nota signos como dolor en el ojo o aumento de las molestias en el ojo, si empeora el enrojecimiento en el ojo, visión borrosa o disminución de la visión, un aumento del número de pequeñas manchas en la visión o aumento de la sensibilidad a la luz.
- En algunos pacientes, después de la inyección la presión en el ojo puede aumentar durante un corto periodo de tiempo. Es posible que usted no se de cuenta de ello, por lo que puede que su médico le realice un seguimiento de la presión ocular después de cada inyección.
- Informe a su médico si ha tenido enfermedades en los ojos o ha recibido algún tratamiento en los ojos anteriormente, o si ha sufrido un accidente cerebrovascular o ha tenido signos pasajeros de accidente cerebrovascular (debilidad o parálisis de un miembro o cara, dificultad en el habla o en la comprensión). Esta información se tendrá en consideración para evaluar si Ximluci es el tratamiento apropiado para usted.
Para consultar información más detallada sobre los efectos adversos que podrían ocurrir durante el tratamiento con Ximluci, ver sección 4 (“Posibles efectos adversos”).
Niños y adolescentes (menores de 18 años)
No se recomienda el uso de Ximluci en niños y adolescentes, ya que no se ha establecido en estos grupos de edad.
Otros medicamentos y Ximluci
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
- Las mujeres que pudieran quedarse embarazadas deben utilizar un método anticonceptivo eficaz durante el tratamiento y durante al menos los tres meses posteriores a la última inyección de Ximluci.
- No hay experiencia en el uso de Ximluci en mujeres embarazadas. Ximluci no se debe usar durante el embarazo salvo que el beneficio potencial supere el riesgo potencial para el feto. Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes del tratamiento con Ximluci.
- No se recomienda el uso de Ximluci durante la lactancia, porque se desconoce si Ximluci pasa a la leche materna. Consulte a su médico o farmacéutico antes del tratamiento con Ximluci.
Conducción y uso de máquinas
Después del tratamiento con Ximluci usted puede experimentar visión borrosa temporalmente. Si esto le ocurre, no conduzca ni use máquinas hasta que este síntoma desaparezca.
3. Cómo se administra Ximluci
Ximluci se administra por el oftalmólogo en forma de inyección única en el ojo bajo anestesia local. La dosis habitual de una inyección es 0,05 ml (que contiene 0,5 mg de principio activo). El intervalo entre dos dosis aplicadas en el mismo ojo debe ser como mínimo de cuatro semanas. Todas las inyecciones serán administradas por un oftalmólogo.
Para prevenir una infección, antes de la inyección su médico le lavará el ojo cuidadosamente. Su médico también le administrará un anestésico local para reducir o prevenir cualquier dolor que pudiera sentir con la inyección.
El tratamiento se inicia con una inyección de Ximluci cada mes. Su médico controlará la enfermedad de su ojo y dependiendo de cómo responda al tratamiento, decidirá si necesita o no recibir más tratamiento y cuándo necesita ser tratado.
Al final del prospecto en el apartado "Cómo preparar y administrar Lucentis en adultos" se dan instrucciones detalladas de uso”.
Pacientes de edad avanzada (65 años y mayores)
Ximluci puede utilizarse en personas de 65 años de edad o más, y no es necesario un ajuste de la dosis.
Antes de interrumpir el tratamiento con Ximluci
Si usted se está planteando interrumpir el tratamiento con Ximluci, acuda a la siguiente consulta y coméntelo antes con su médico. Su médico le aconsejará y decidirá durante cuánto tiempo deberá ser tratado con Ximluci.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos asociados con la administración de Ximluci se deben o al propio medicamento o al procedimiento de inyección y la mayoría afectan al ojo.
Efectos adversos graves:
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
- desprendimiento o desgarro de una capa en la parte interna del ojo (desprendimiento o desgarro de la retina), que da como resultado destellos de luz con partículas flotantes que progresan a una pérdida de visión transitoria o a un enturbiamiento del cristalino (catarata)
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes)
- ceguera,
- infección del globo ocular (endoftalmitis) con inflamación de la parte interna del ojo
Los síntomas que podría experimentar son:
- dolor o aumento de las molestias en el ojo
- empeoramiento del enrojecimiento en el ojo
- visión borrosa o disminución de la visión
- un aumento del número de pequeñas manchas en la visión
- aumento de la sensibilidad a la luz
Informe a su médico inmediatamente si presenta alguno de estos efectos adversos.
Otros efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
Los efectos adversos oculares incluyen
- inflamación del ojo
- sangrado en la parte posterior del ojo (hemorragia en la retina)
- alteraciones visuales
- dolor en el ojo
- pequeñas partículas o manchas en la visión (partículas flotantes)
- sangre en el ojo
- irritación del ojo
- sensación de tener algo dentro del ojo
- aumento de la producción de lágrima
- inflamación o infección en el borde de los párpados
- ojo seco
- enrojecimiento o picor en el ojo
- aumento de la presión en el ojo
Los efectos adversos no oculares incluyen
- dolor de garganta, congestión nasal, goteo nasal
- dolor de cabeza
- dolor en las articulaciones
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
Los efectos adversos oculares incluyen
- disminución de la nitidez de la visión
- hinchazón de una sección del ojo (úvea, córnea)
- inflamación de la córnea (parte delantera del ojo)
- pequeñas marcas en la superficie del ojo
- visión borrosa
- sangrado en el lugar de inyección
- sangrado en el ojo
- secreción del ojo con picor enrojecimiento e hinchazón (conjuntivitis)
- sensibilidad a la luz
- molestias en el ojo
- hinchazón del párpado
- dolor en el párpado
Los efectos adversos no oculares incluyen
- infección de las vías urinarias
- recuento de glóbulos rojos bajo (con síntomas tales como cansancio, dificultad al respirar, mareo, palidez)
- ansiedad
- tos
- náuseas, reacciones alérgicas tales como erupción, urticaria, picor y enrojecimiento de la piel
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes)
Los efectos adversos oculares incluyen
- inflamación y sangrado en la parte anterior del ojo
- acúmulo de pus en el ojo
- cambios en la parte central de la superficie ocular
- dolor o irritación en el lugar de inyección
- sensación anormal en el ojo
- irritación del párpado
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ximluci
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta del vial después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2ºC y 8ºC). No congelar.
- Antes de usar, el vial sin abrir se puede conservar a temperatura ambiente (25ºC) durante un máximo de 48 horas.
- Conservar el vial en el embalaje exterior para protegerlo de la luz.
- No utilice ningún envase que esté dañado.
6. Contenido del envase e información adicional
Composición de Ximluci
- El principio activo es ranibizumab. Cada ml contiene 10 mg de ranibizumab. Cada vial contiene 2,3 mg de ranibizumab en 0,23 ml de solución. Esto aporta una cantidad utilizable para proporcionar una dosis única de 0,05 ml, que contiene 0,5 mg de ranibizumab.
- Los demás componentes son trehalosa dihidrato; hidrocloruro de histidina monohidrato; histidina; polisorbato 20; agua para inyectables.
Aspecto del producto y contenido del envase
Ximluci es una solución inyectable de transparente a ligeramente opalescente, de incolora a ligeramente amarronada contenida en un vial (0,23 ml).
Se dispone de dos tipos de envase diferentes:
Envase solo con vial
Envase que contiene un vial de vidrio con ranibizumab, con tapón de goma de bromobutil. El vial es para un solo uso.
Envase de vial + aguja con filtro
Envase que contiene un vial de vidrio con ranibizumab, con tapón de goma de bromobutil y una aguja estéril roma con filtro de 5 µm (18G x 1½″, 1,2 mm x 40 mm) para extraer el contenido del vial.
Todos los componentes son para un solo uso.
Titular de la autorización de comercialización y responsable de la fabricación
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Germany
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien EG (Eurogenerics) NV Tél/Tel: + 32 4797878 | Lietuva UAB „STADA Baltics“ Tel: + 370 52603926 |
STADA Bulgaria EOOD Te?.: + 359 29624626 | Luxembourg/Luxemburg EG (Eurogenerics) NV Tél/Tel: + 32 4797878 |
Ceská republika STADA PHARMA CZ s.r.o. Tel: + 420 257888111 | Magyarország STADA Hungary Kft Tel.: + 36 18009747 |
Danmark STADA Nordic ApS Tlf: + 45 44859999 | Malta Pharma MT Ltd Tel: + 356 21337008 |
Deutschland STADAPHARM GmbH Tel: + 49 61016030 | Nederland Centrafarm B.V. Tel.: + 31 765081000 |
Eesti UAB „STADA Baltics“ Tel: + 370 52603926 | Norge STADA Nordic ApS Tlf: + 45 44859999 |
Ελλáδα STADA Arzneimittel AG Τηλ: +30 2106664667 | Österreich STADA Arzneimittel GmbH Tel: + 43 136785850 |
España Laboratorio STADA, S.L. Tel: + 34 934738889 | Polska STADA Poland Sp. z.o o. Tel: + 48 227377920 |
France EG LABO - Laboratoires EuroGenerics Tél: + 33 146948686 | Portugal Stada, Lda. Tel: + 351 211209870 |
Hrvatska STADA d.o.o. Tel: + 385 13764111 | România STADA M&D SRL Tel: + 40 213160640 |
Ireland Clonmel Healthcare Ltd. Tel: + 353 526177777 | Slovenija Stada d.o.o. Tel: + 386 15896710 |
Ísland STADA Arzneimittel AG Sími: + 49 61016030 | Slovenská republika STADA PHARMA Slovakia, s.r.o. Tel: + 421 252621933 |
Italia EG SpA Tel: + 39 028310371 | Suomi/Finland STADA Nordic ApS, Suomen sivuliike Puh/Tel: + 358 207416888 |
Κúπρος STADA Arzneimittel AG Τηλ: +30 2106664667 | Sverige STADA Nordic ApS Tel: + 45 44859999 |
Latvija UAB „STADA Baltics“ Tel: + 370 52603926 | United Kingdom (Northern Ireland) STADA Arzneimittel AG Tel: +49 61016030 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
ESTA INFORMACIÓN ESTÁ DESTINADA ÚNICAMENTE A PROFESIONALES SANITARIOS:
Ver también la sección 3 “Cómo se administra Ximluci”.
Cómo preparar y administrar Ximluci en adultos
Vial para un solo uso. Únicamente para vía intravítrea.
Ximluci debe ser administrado por un oftalmólogo que tenga experiencia en la administración de inyecciones intravítreas.
En la DMAE exudativa, en la NVC, en RDP y en la alteración visual debida a EMD o a edema macular secundario a OVR la dosis recomendada de Ximluci es 0,5 mg administrada en forma de inyección intravítrea única. Esto corresponde a un volumen de inyección de 0,05 ml. El intervalo entre dos dosis inyectadas en el mismo ojo debe ser como mínimo de cuatro semanas.
El tratamiento se inicia con una inyección al mes hasta alcanzar la agudeza visual máxima y/o no haya signos de actividad de la enfermedad, es decir ningún cambio en la agudeza visual ni en otros signos y síntomas de la enfermedad bajo tratamiento continuado. En pacientes con DMAE exudativa, EMD, RDP y OVR inicialmente pueden ser necesarias tres o más inyecciones consecutivas administradas mensualmente.
A partir de ese momento, los intervalos de monitorización y tratamiento se deben determinar según criterio médico y en base a la actividad de la enfermedad, valorada mediante la agudeza visual y/o parámetros anatómicos.
Se debe interrumpir el tratamiento con Ximluci si bajo criterio del médico, los parámetros visuales y anatómicos indican que el paciente no se está beneficiando del tratamiento continuado.
La monitorización para determinar la actividad de la enfermedad puede incluir examen clínico, control funcional o técnicas de imagen (p. ej. tomografía de coherencia óptica o angiografía con fluoresceína).
Si se está tratando a los pacientes de acuerdo a un régimen de tratar y extender, una vez se ha alcanzado la agudeza visual máxima y/o no hay signos de actividad de la enfermedad, los intervalos de tratamiento se pueden espaciar de forma gradual hasta que vuelvan a aparecer signos de actividad de la enfermedad o alteración visual. En el caso de la DMAE exudativa el intervalo de tratamiento no debe espaciarse en más de dos semanas cada vez y en el caso del EMD se puede espaciar hasta un mes cada vez. Para la RDP y la OVR, los intervalos de tratamiento también pueden espaciarse de forma gradual, sin embargo los datos que hay no son suficientes para determinar la duración de estos intervalos. Si vuelve a aparecer actividad de la enfermedad, se debe acortar el intervalo de tratamiento de manera consecuente.
El tratamiento de la alteración visual debida a NVC se debe determinar para cada paciente de forma individualizada en base a la actividad de la enfermedad. Algunos pacientes pueden necesitar sólo una inyección durante los primeros 12 meses; otros pueden necesitar tratamiento con mayor frecuencia, incluyendo una inyección mensual. En el caso de NVC secundaria a miopía patológica (MP), muchos pacientes pueden necesitar sólo una o dos inyecciones durante el primer año.
Ximluci y fotocoagulación con láser en EMD y edema macular secundario a oclusión de la rama venosa retiniana (ORVR)
Existe alguna experiencia con Ximluci administrado concomitantemente con fotocoagulación con láser. Cuando se administren en el mismo día, Ximluci debe ser administrado como mínimo
30 minutos después de la fotocoagulación con láser. Ximluci puede administrarse en pacientes que han recibido fotocoagulación con láser previamente.
Ximluci y la terapia fotodinámica con verteporfina en la NVC secundaria a MP
No hay experiencia en la administración concomitante de Ximluci y verteporfina.
Antes de la administración de Ximluci se debe comprobar visualmente la ausencia de partículas y decoloración.
El procedimiento de inyección deberá llevarse a cabo bajo condiciones asépticas, que incluyen el lavado quirúrgico de las manos, el uso de guantes estériles, un campo estéril, un blefarostato estéril para los párpados (o equivalente) y la disponibilidad de una paracentesis estéril (en caso necesario). Antes de realizar el procedimiento de inyección intravítrea, se deberá evaluar detalladamente la historia clínica del paciente en cuanto a reacciones de hipersensibilidad. Antes de la inyección se debe administrar una anestesia adecuada y un microbicida tópico de amplio espectro para desinfectar la piel de la zona periocular, párpado y superficie ocular, de acuerdo con la práctica local.
Envase solo con vial
El vial es para un solo uso. Tras la inyección se debe desechar cualquier sobrante de producto no utilizado. No se debe utilizar ningún vial que muestre signos de deterioro o manipulación. La esterilidad sólo se puede garantizar si el sellado del envase se mantiene intacto.
Para la preparación y la inyección intravítrea se necesitan los siguientes productos sanitarios (para un solo uso):
- una aguja estéril con filtro de 5 µm (18G x 1½″, 1.2 mm x 40 mm)
- una jeringa estéril de 1 ml (que incluya una marca en 0,05 ml)
- una aguja para inyección (30G x ½″, 0.3 mm x 13 mm).
Estos productos sanitarios no se incluyen en el envase de Ximluci.
Envase de vial + aguja con filtro
Todos los componentes son estériles y para un solo uso. No se debe utilizar ningún componente cuyo envase muestre signos de deterioro o manipulación. La esterilidad sólo se puede garantizar si el sellado del envase de los componentes se mantiene intacto. La reutilización puede dar lugar a una infección u otra enfermedad/lesión.
Para la preparación y la inyección intravítrea se necesitan los siguientes productos sanitarios (para un solo uso):
- una aguja estéril con filtro de 5 µm (18G x 1½″, 1,2 mm x 40 mm, suministrada)
- una jeringa estéril de 1 ml (que incluya una marca en 0,05 ml, no incluida en el envase de Ximluci)
- una aguja para inyección (30G x ½″, 0.3 mm x 13 mm; no incluida en el envase de Ximluci)
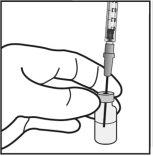
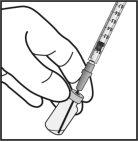
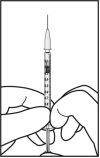
Para la preparación de Ximluci para administración intravítrea en pacientes adultos, siga las siguientes instrucciones:
|
|
|
|
Nota: Sujetar la aguja para inyección por el cono mientras se retira la cápsula de cierre. |
|
Nota: No secar la aguja para inyección. No tirar del émbolo hacia atrás. |
|
La aguja para inyección se deberá introducir 3,5-4,0 mm por detrás del limbo en la cavidad vítrea, evitando el meridiano horizontal y en dirección al centro del globo. Seguidamente debe liberarse el volumen de inyección de 0,05 ml; las inyecciones siguientes deberán aplicarse cada vez en un punto escleral distinto.
Tras la inyección, no tapar la aguja con la cápsula de cierre ni separarla de la jeringa. Eliminar la jeringa usada junto con la aguja en un contenedor para objetos punzantes o eliminar de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a XIMLUCI 10 MG/ML SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 10 MG/MLPrincipio activo: RanibizumabFabricante: Samsung Bioepis Nl B.V.Requiere recetaForma farmacéutica: INYECTABLE, 10 MG/MLPrincipio activo: RanibizumabFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: INYECTABLE, 10 MG/MLPrincipio activo: RanibizumabFabricante: Novartis Europharm LimitedRequiere receta
Médicos online para XIMLUCI 10 MG/ML SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de XIMLUCI 10 MG/ML SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes