XEPLION 150 mg SUSPENSION INYECTABLE DE LIBERACION PROLONGADA

Cómo usar XEPLION 150 mg SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Xeplion 25 mg suspensión inyectable de liberación prolongada
Xeplion 50 mg suspensión inyectable de liberación prolongada
Xeplion 75 mg suspensión inyectable de liberación prolongada
Xeplion 100 mg suspensión inyectable de liberación prolongada
Xeplion 150 mg suspensión inyectable de liberación prolongada
Paliperidona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Xeplion y para qué se utiliza
- Qué necesita saber antes de empezar a usar Xeplion
- Cómo usar Xeplion
- Posibles efectos adversos
- Conservación de Xeplion
- Contenido del envase e información adicional
1. Qué es Xeplion y para qué se utiliza
Xeplion contiene el principio activo paliperidona que pertenece a la clase de medicamentos antipsicóticos y se utiliza como tratamiento de mantenimiento para los síntomas de esquizofrenia en pacientes adultos estabilizados con paliperidona o risperidona.
Si usted ha mostrado respuesta a paliperidona o risperidona en el pasado y tiene síntomas leves o moderados, su médico puede iniciar el tratamiento con Xeplion sin una estabilización previa con paliperidona o risperidona.
La esquizofrenia es un trastorno con síntomas "positivos" y "negativos". Positivo significa un exceso de síntomas que normalmente no están presentes. Por ejemplo, una persona con esquizofrenia puede escuchar voces o ver cosas que no existen (denominadas alucinaciones), tener creencias erróneas (denominadas delirios) o tener una desconfianza en los demás fuera de lo normal. Negativo se refiere a la falta de conductas o sentimientos que normalmente están presentes. Por ejemplo, una persona con esquizofrenia puede encerrarse en sí misma y no responder a ningún estímulo emocional o pueden tener problemas para hablar de una manera clara y lógica. Las personas que padecen este trastorno también pueden sentirse deprimidas, ansiosas, culpables o tensas.
Xeplion puede ayudar a aliviar los síntomas de su enfermedad y evitar que reaparezcan.
2. Qué necesita saber antes de empezar a usar Xeplion
No use Xeplion
- si es alérgico a paliperidona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico a cualquier otro medicamento antipsicótico, incluida la risperidona.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Xeplion.
Este medicamento no ha sido estudiado en pacientes de edad avanzada con demencia. Sin embargo, los pacientes de edad avanzada con demencia que están siendo tratados con otros medicamentos similares, pueden ver aumentado el riesgo de ataque cerebral o muerte (ver sección 4, posibles efectos adversos).
Todos los medicamentos tienen efectos secundarios y algunos de los efectos secundarios de este medicamento pueden empeorar los síntomas de otras patologías. Por esa razón, es importante que comente con su médico cualquiera de las siguientes enfermedades, que podrían empeorar durante el tratamiento con este medicamento:
- si tiene la enfermedad de Parkinson
- si alguna vez ha sido diagnosticado de una enfermedad cuyos síntomas incluyan temperatura elevada y rigidez muscular (también conocida como Síndrome Neuroléptico Maligno)
- si alguna vez ha experimentado movimientos anómalos de la lengua o cara (Discinesia Tardía)
- si ha tenido en el pasado niveles bajos de células blancas de la sangre (que puede o no haber sido causado por otros medicamentos)
- si es diabético o tiene tendencia a la diabetes
- si ha tenido cáncer de mama o un tumor en la hipófisis del cerebro
- si padece alguna enfermedad cardiaca o si recibe tratamiento para enfermedades cardiacas que pueden hacerle más propenso a una reducción de la tensión arterial
- si tiene la tensión arterial baja cuando se pone de pie o se incorpora de forma repentina
- si padece epilepsia
- si tiene problemas de riñón
- si tiene problemas de hígado
- si tiene una erección prolongada y/o dolorosa
- si tiene dificultades para el control de la temperatura corporal o está acalorado
- si tiene un nivel anormalmente alto de la hormona prolactina en sangre o si tiene un tumor que posiblemente sea dependiente de la prolactina
- si usted o alguien en su familia tiene antecedentes de coágulos sanguíneos, dado que los antipsicóticos se han asociado con la formación de coágulos de sangre.
Si tiene alguna de estas enfermedades, por favor consulte con su médico ya que podría ser necesario un ajuste de su dosis o mantenerle en observación durante un tiempo.
Debido a que en muy raras ocasiones se ha observado en pacientes tratados con este medicamento un número peligrosamente bajo de un tipo de células blancas necesarias para combatir las infecciones en la sangre, su médico puede comprobar el número de células blancas de la sangre.
Incluso si usted ha tolerado previamente paliperidona oral o risperidona, raramente ocurren reacciones alérgicas después de recibir inyecciones de Xeplion. Solicite ayuda médica inmediatamente si experimenta una erupción cutánea, hinchazón de la garganta, picor o problemas de la respiración, ya que éstos pueden ser signos de una reacción alérgica grave.
Este medicamento puede hacerle aumentar de peso. Un aumento de peso significativo puede afectar negativamente a su salud. Su médico realizará regularmente un seguimiento de su peso.
En pacientes tratados con este medicamento se ha observado diabetes mellitus o empeoramiento de diabetes mellitus preexistente, su médico debe comprobar los signos de un aumento de azúcar en sangre. En pacientes con diabetes mellitus preexistente se debe monitorizar regularmente el azúcar en sangre.
Dado que este medicamento puede reducir el impulso de vomitar, existe la posibilidad de que pueda enmascarar la respuesta normal del organismo ante la ingestión de sustancias tóxicas u otras patologías.
Durante la intervención en el ojo por turbidez de las lentes (cataratas), la pupila (el círculo negro situado en medio del ojo), puede no aumentar de tamaño como se necesita. Además, el iris (la parte coloreada del ojo) puede ponerse flácido durante la cirugía y esto puede causar daño en el ojo. Si usted está pensando en ser operado de los ojos, asegúrese de informar a su oftalmólogo que está usando este medicamento.
Niños y adolescentes
No utilizar este medicamento en menores de 18 años.
Uso de Xeplion con otros medicamentos
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
La toma de este medicamento junto con carbamazepina (un anti-epiléptico y estabilizador del ánimo) puede requerir un cambio de su dosis de este medicamento.
Dado que este medicamento actúa principalmente en el cerebro, la interacción con otros medicamentos que también actúen sobre él puede causar una exageración de los efectos secundarios, tales como somnolencia u otros efectos sobre el cerebro tales como otros medicamentos psiquiátricos, opioides, antihistamínicos y medicamentos para dormir.
Dado que este medicamento puede reducir la tensión arterial, deberá tener cuidado si usa este medicamento con otros medicamentos que también la reduzcan.
Este medicamento puede reducir el efecto de los medicamentos para la enfermedad de Parkinson y el síndrome de piernas inquietas (p. ej., levodopa).
Este medicamento puede causar una anomalía en el electrocardiograma (ECG) que pone de manifiesto que es necesario un período prolongado para que un impulso eléctrico viaje a través de una cierta parte del corazón (conocido como "prolongación del intervalo QT"). Otros medicamentos que tienen este efecto incluyen algunos medicamentos usados para tratar el ritmo cardíaco o para tratar las infecciones, además de otros antipsicóticos.
Si es propenso a desarrollar convulsiones, es posible que este medicamento aumente sus probabilidades de experimentarlas. Otros medicamentos que tienen este efecto incluyen algunos medicamentos usados para tratar la depresión o para tratar las infecciones, además de otros antipsicóticos.
Xeplion se debe usar con precaución con medicamentos que aumentan la actividad del sistema nervioso central (psicoestimulantes tales como metilfenidato).
Xeplion con alcohol
Se debe evitar el alcohol.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No debe usar este medicamento durante el embarazo a menos que lo haya comentado con su médico. Se pueden producir los siguientes síntomas en bebés recién nacidos, de madres que han sido tratadas con paliperidona en el último trimestre de embarazo (últimos tres meses de su embarazo): temblor, rigidez y/o debilidad muscular, somnolencia, agitación, problemas al respirar y dificultad en la alimentación. Si su bebé desarrolla cualquiera de estos síntomas se debe poner en contacto con su médico.
Este medicamento puede pasar de madre a hijo por la leche materna y puede dañar al bebé. Por consiguiente, no debe dar el pecho mientras esté usando este medicamento.
Conducción y uso de máquinas
Durante el tratamiento con este medicamento pueden aparecer mareos, cansancio extremo y problemas de la visión (ver sección 4). Esto debe tenerse en cuenta cuando se requiera una atención máxima, p. ej., cuando conduzca o maneje máquinas.
Xeplion contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio".
3. Cómo usar Xeplion
Su médico u otro profesional sanitario le administrará este medicamento. Su médico le indicará cuándo se debe administrar la siguiente inyección. Es importante que no omita ninguna de las dosis programadas. Si no puede asistir a su cita con el médico, asegúrese de llamarle de inmediato para concertar otra cita tan pronto como sea posible.
Recibirá la primera inyección (150 mg) y la segunda inyección (100 mg) de este medicamento en la parte superior del brazo aproximadamente con una semana de diferencia. A partir de entonces, recibirá una inyección (de entre 25 mg y 150 mg) en la parte superior del brazo o en las nalgas una vez al mes.
Si su médico le está cambiando de risperidona inyectable de larga duración a este medicamento, recibirá la primera inyección de este medicamento (de entre 25 mg y 150 mg) en la parte superior del brazo o en las nalgas en la próxima inyección programada. A partir de entonces, recibirá una inyección (de entre 25 mg y 150 mg) en la parte superior del brazo o en las nalgas una vez al mes.
Dependiendo de sus síntomas, el médico puede aumentar o disminuir la cantidad de medicamento que recibe en un nivel de dosis en el momento de la inyección mensual programada.
Pacientes con problemas de riñón
Su médico puede ajustar la dosis de este medicamento de acuerdo a su función renal. Si usted tiene problemas leves de riñón, su médico le puede dar una dosis menor. No deberá usar este medicamento si usted tiene problemas de riñón moderados o graves.
Pacientes de edad avanzada
Su médico puede reducir la dosis de este medicamento si la función de su riñón está disminuída.
Si recibe más Xeplion del que debiera
Recibirá este medicamento bajo supervisión médica; es, por tanto, poco probable que reciba una dosis excesiva.
Los pacientes que hayan recibido un exceso de paliperidona pueden experimentar los síntomas siguientes: somnolencia o sedación, frecuencia cardiaca rápida, tensión arterial baja, anomalías en el electrocardiograma (trazado eléctrico del corazón), o movimientos lentos o anómalos de la cara, el cuerpo, los brazos o las piernas.
Si interrumpe el tratamiento con Xeplion
Si deja de recibir sus inyecciones, se perderán los efectos del medicamento. No debe dejar de usar este medicamento a menos que se lo indique su médico ya que podrían aparecer de nuevo los síntomas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe inmediatamente a su médico si:
- presenta coágulos sanguíneos en las venas, especialmente en las piernas (los síntomas incluyen hinchazón, dolor y enrojecimiento de la pierna), que pueden circular a través de los vasos sanguíneos a los pulmones causando dolor en el pecho y dificultad al respirar. Si usted nota alguno de estos síntomas pida consejo médico inmediatamente.
- tiene demencia y presenta un cambio repentino de su estado mental o debilidad repentina o entumecimiento de la cara, brazos o piernas especialmente en uno de los lados, o le cuesta hablar incluso durante un periodo corto de tiempo. Pueden ser signos de un infarto cerebral.
- presenta fiebre, rigidez muscular, sudoración o una disminución del nivel de consciencia (trastorno conocido como “Síndrome Neuroléptico Maligno”). Puede necesitar tratamiento médico inmediato.
- es hombre y presenta una erección prolongada o dolorosa. Se conoce como priapismo. Puede necesitar tratamiento médico inmediato.
- presenta movimientos rítmicos involuntarios de la lengua, boca y cara. Puede ser necesario la retirada de paliperidona.
- presenta una reacción alérgica grave caracterizada por fiebre, hinchazón de la boca, la cara, los labios o la lengua, dificultad respiratoria, picores, erupción cutánea y algunas veces descenso de la tensión arterial (es decir, una "reacción anafiláctica"). Incluso si previamente ha tolerado risperidona oral o paliperidona oral, en raras ocasiones han aparecido reacciones alérgicas después de recibir las inyecciones de paliperidona.
- tiene previsto someterse a una operación en el ojo, asegúrese de decirle a su oftalmólogo que está tomando este medicamento. Durante una operación del ojo por opacidad de las lentes (cataratas), es posible que el iris (la parte coloreada del ojo) quede flácido durante la cirugía (lo que se denomina “síndrome del iris flácido”) lo que puede ocasionar daño en el ojo.
- presenta un número peligrosamente bajo de un tipo de células blancas de la sangre necesarias para combatir las infecciones sanguíneas.
Pueden aparecer los siguientes efectos adversos:
Efectos adversos muy frecuentes: pueden afectar a más de 1 de cada 10 pacientes
- dificultad para quedarse o permanecer dormido.
Efectos adversos frecuentes: pueden afectar hasta 1 de cada 10 pacientes
- síntomas de resfriado común, infección del tracto urinario, sentir como si tuviese gripe
- Xeplion puede aumentar los niveles de una hormona llamada “prolactina” que se detecta en los análisis de sangre (lo cual puede o no causar síntomas). Cuando aparecen los síntomas del aumento de la prolactina, pueden incluir (en hombres) hinchazón de los pechos, dificultad en tener o mantener erecciones u otras disfunciones sexuales; (en mujeres) malestar de las mamas, secreción de leche por las mamas, pérdida de períodos menstruales u otros problemas con el ciclo.
- aumento del azúcar en sangre, aumento de peso, pérdida de peso, apetito disminuido
- irritabilidad, depresión, ansiedad
- parkinsonismo: Esta enfermedad puede incluir movimiento lento o alterado, sensación de rigidez o tirantez de los músculos (haciendo movimientos bruscos) y algunas veces una sensación de “congelación” del movimiento que después se reinicia. Otros signos del parkinsonismo incluyen caminar despacio arrastrando los pies, temblor mientras descansa, aumento de la saliva y/o babear y pérdida de expresividad de la cara
- inquietud, sentirse somnoliento o menos atento
- distonía: Es un trastorno que implica contracción involuntaria lenta o continua de los músculos. Aunque puede estar afectada cualquier parte del cuerpo (y puede originar posturas anormales), la distonía afecta con frecuencia a los músculos de la cara, incluyendo movimientos anormales de los ojos, boca, lengua o mandíbula
- mareos
- discinesia: Este trastorno implica movimientos musculares involuntarios y puede incluir movimientos repetitivos, espasmódicos o de retorcimiento, o espasmos
- temblor (agitación)
- dolor de cabeza
- latido rápido del corazón
- aumento de la presión arterial
- tos, congestión nasal
- dolor abdominal, vómitos, náuseas, estreñimiento, diarrea, indigestión, dolor de muelas
- aumento de las transaminasas del hígado en sangre
- dolor de huesos o músculos, dolor de espalda, dolor de las articulaciones
- ausencia de menstruación
- secreción de leche de los pechos
- fiebre, debilidad, fatiga (cansancio)
- una reacción en el lugar de la inyección, incluyendo picor, dolor o hinchazón.
Efectos adversos poco frecuentes: pueden afectar hasta 1 de cada 100 pacientes
- neumonía, infección de pecho (bronquitis), infección de las vías respiratorias, infección de nariz, infección de vejiga, infección de oídos, infección de las uñas por hongos, amigdalitis, infección de la piel
- disminución del número de células blancas de la sangre, disminución de un tipo de células blancas de la sangre que ayudan a combatir las infecciones, disminución de las plaquetas (células de la sangre que ayudan a detener las hemorragias), anemia
- reacción alérgica
- diabetes o empeoramiento de la diabetes, aumento de la insulina (una hormona que controla los niveles de azúcar en sangre) en sangre
- aumento de apetito
- pérdida de apetito que causa malnutrición y disminución del peso corporal
- aumento de los triglicéridos en sangre (grasa), aumento del colesterol en sangre
- trastorno del sueño, euforia (manía), disminución del deseo sexual, nerviosismo, pesadillas
- discinesia tardía (espasmos o movimientos espasmódicos que no se pueden controlar en la cara, lengua u otras partes del cuerpo). Informe a su médico inmediatamente si experimenta movimientos rítmicos involuntarios de la lengua, boca o cara. Puede ser necesaria la retirada de este medicamento.
- desmayo, una inquietud que provoca el movimiento de partes del cuerpo, mareos al ponerse de pie, alteración de la atención, problemas con el habla, pérdida o alteraciones del gusto, disminución de la sensibilidad de la piel al dolor o al tacto, sensación de hormigueo, pinchazos o entumecimiento de la piel
- visión borrosa, infección de ojos u “ojo rojo”, sequedad de ojos
- sensación de que todo gira (vértigo), pitidos en los oídos, dolor de oídos
- interrupción de la conducción entre las partes superiores e inferiores del corazón, anomalía en la actividad eléctrica del corazón, prolongación del intervalo QT en el corazón, latido rápido del corazón al ponerse de pie, latido lento del corazón, anomalías en el trazado eléctrico del corazón (electrocardiograma o ECG), sensación de aleteo o de golpeteo en el pecho (palpitaciones)
- disminución de la presión arterial, presión arterial baja al ponerse de pie (por lo tanto, algunas personas que toman este medicamento pueden sentirse débiles, mareadas o pueden desmayarse cuando se levantan o se sientan de repente)
- respiración entrecortada, congestión de las vías respiratorias, jadeo, dolor de garganta, sangrados nasales
- malestar abdominal, infección de estómago o de intestino, dificultad al tragar, sequedad de boca
- exceso de gas o flatulencia
- aumento de la GGT (una enzima del hígado llamada gamma-glutamiltransferasa) en sangre, aumento de las enzimas del hígado en sangre
- ronchas ( o “urticaria”), picor, erupción, caída del pelo, eccema, sequedad de la piel, enrojecimiento de la piel, acné
- aumento de la CPK (creatina fosfoquinasa) en sangre, una enzima que algunas veces se libera con la degradación muscular
- espasmos musculares, rigidez de las articulaciones, debilidad muscular, dolor de cuello
- incontinencia (pérdida de control) urinaria, orinar con frecuencia, dolor al orinar
- disfunción eréctil, trastorno de la eyaculación, ausencia de períodos menstruales u otros problemas con el ciclo (mujeres), desarrollo de senos en los hombres, disfunción sexual, dolor en las mamas
- hinchazón de la cara, boca, ojos o labios, hinchazón del cuerpo, brazos o piernas
- aumento de la temperatura corporal
- cambio en la forma de andar
- dolor de pecho, malestar de pecho, sensación de malestar
- endurecimiento de la piel
- caídas.
Efectos adversos raros: pueden afectar hasta 1 de cada y 1.000 pacientes
- infección de ojos
- inflamación de la piel causada por ácaros, absceso bajo la piel
- aumento de eosinófilos (un tipo de célula blanca de la sangre) en la sangre
- secreción inadecuada de la hormona que controla el volumen de orina
- azúcar en la orina
- complicaciones de la diabetes no controlada potencialmente mortales
- disminución del azúcar en sangre
- ingesta excesiva de agua
- falta de movimiento o de respuesta estando despierto (catatonía)
- confusión
- sonambulismo
- ausencia de emociones
- incapacidad para conseguir el orgasmo
- síndrome neuroléptico maligno (confusión, disminución o pérdida de consciencia, fiebre alta y rigidez muscular grave), problemas en los vasos sanguíneos del cerebro, que incluyen pérdida repentina del riego de sangre al cerebro (ictus o “mini” ictus), sin respuesta a estímulos, pérdida de la consciencia, disminución del nivel de consciencia, convulsiones (crisis epilépticas), trastorno del equilibrio
- coordinación anormal
- glaucoma (aumento de la presión del globo ocular)
- problemas de movimientos de los ojos, giro de los ojos, hipersensibilidad de los ojos a la luz, aumento del lagrimeo, enrojecimiento de los ojos
- fibrilación auricular (ritmo cardíaco anormal), latido irregular del corazón
- coágulos de sangre en las venas especialmente en las piernas (los síntomas incluyen hinchazón, dolor y enrojecimiento de la pierna). Si usted sufre alguno de estos síntomas solicite ayuda médica inmediatamente
- rubor
- dificultad para respirar durante el sueño (apnea del sueño)
- congestión pulmonar
- ruidos crepitantes de los pulmones
- inflamación del páncreas, hinchazón de la lengua, incontinencia fecal, heces muy duras
- labios agrietados
- erupción en la piel relacionada con el medicamento, engrosamiento de la piel, caspa
- rotura de las fibras musculares y dolor muscular (rabdomiólisis)
- hinchazón de las articulaciones
- incapacidad para orinar
- malestar en las mamas, crecimiento de las glándulas de las mamas, crecimiento de la mama
- secreción vaginal
- temperatura corporal muy baja, escalofríos, sensación de sed,
- síntomas de abstinencia al medicamento
- acumulación de pus debido a una infección en el lugar de la inyección, infección profunda de la piel, quiste en el lugar de la inyección, moratón en el lugar de la inyección.
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles
- número peligrosamente bajo de un tipo células blancas de la sangre necesarias para combatir infecciones
- reacción alérgica grave caracterizada por fiebre, hinchazón de la boca, cara, labios o lengua, dificultad respiratoria, picores, erupción cutánea y algunas veces descenso de la presión arterial
- ingesta de agua peligrosamente excesiva
- trastorno alimentario relacionado con el sueño
- coma debido a diabetes no controlada
- agitación de la cabeza
- coágulos de sangre en los pulmones provocando dolor en el pecho y dificultad respiratoria. Si usted sufre alguno de estos síntomas acuda al médico inmediatamente.
- disminución del oxígeno en partes del cuerpo (debido a la disminución del flujo sanguíneo)
- respiración rápida, superficial, neumonía causada por aspiración de alimentos, trastorno de la voz
- obstrucción intestinal, ausencia del movimiento del intestino que provoca obstrucción
- color amarillo de la piel y los ojos (ictericia)
- reacción alérgica grave con hinchazón que puede afectar a la garganta, causando dificultad respiratoria
- decoloración de la piel, piel o cuero cabelludo escamoso y con picor
- anomalía en la postura
- bebés recién nacidos, de madres que han sido tratadas con Xeplion durante el embarazo pueden experimentar efectos adversos al medicamento y/o síntomas de abstinencia, tales como irritabilidad, contracciones musculares débiles o mantenidas, agitación, somnolencia, problemas al respirar o dificultad en la alimentación
- priapismo (una erección prolongada que puede requerir tratamiento quirúrgico)
- disminución de la temperatura corporal
- células muertas de la piel en el lugar de la inyección y úlcera en el lugar de la inyección.
Comunicación de efectos adversos
Si experimenta cualquier efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Xeplion
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30°C.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Xeplion
El principio activo es paliperidona.
Cada jeringa precargada de Xeplion 25 mg contiene 39 mg de palmitato de paliperidona.
Cada jeringa precargada de Xeplion 50 mg contiene 78 mg de palmitato de paliperidona.
Cada jeringa precargada de Xeplion 75 mg contiene 117 mg de palmitato de paliperidona.
Cada jeringa precargada de Xeplion 100 mg contiene 156 mg de palmitato de paliperidona.
Cada jeringa precargada de Xeplion 150 mg contiene 234 mg de palmitato de paliperidona.
Los demás componentes son:
Polisorbato 20
Polietilenglicol 4000
Ácido cítrico monohidrato
Fosfato ácido disódico anhidro
Fosfato diácido de sodio monohidratado
Hidróxido de sodio (para ajuste del pH)
Agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Xeplion es una suspensión inyectable de liberación prolongada de color blanco a blanquecino, que viene en una jeringa precargada.
Cada envase contiene 1 jeringa precargada y 2 agujas.
Envase de inicio del tratamiento:
Cada envase contiene 1 envase de Xeplion 150 mg y 1 envase de Xeplion 100 mg.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Responsable de la fabricación
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB “JOHNSON & JOHNSON” Tel: +370 5 278 68 88 |
???????? ”??????? & ??????? ????????” ???? ???.:+359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel:+420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.:+36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 45 94 82 82 | Malta AM MANGION LTD. Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137-955-955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB “JOHNSON & JOHNSON” Eesti filiaal Tel.: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλ?δα Janssen-Cilag Φαρμακευτικ? Α.Ε.Β.Ε Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tel: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB C/o Vistor hf Sími: +354 535 7000 | Slovenská republika Johnson & Johnson s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Κ?προς Βαρν?βας Χατζηπαναγ?ς Λτδ Tηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Tel: +46 8 626 50 00 |
Latvija UAB “JOHNSON & JOHNSON” filiale Latvija Tel: +371 6789 3561 | United Kingdom Janssen-Cilag Ltd. Tel: +44 1 494 567 444 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
Esta información está destinada únicamente a médicos o profesionales sanitarios y deben leerla junto con la información de prescripción completa (Resumen de las Características del Producto).
La suspensión inyectable es para un solo uso. Se debe examinar visualmente para detectar cualquier partícula extraña antes de la administración. No use el producto si la jeringa no se encuentra visualmente libre de partículas extrañas.
El envase contiene una jeringa precargada y dos agujas de seguridad (una aguja del calibre 22 de 1½ pulgadas [38,1 mm x 0,72 mm] y una aguja del calibre 23 de 1 pulgada [25,4 mm x 0,64 mm]) para inyección intramuscular. Xeplion también está disponible en forma de un Kit de iniciación del tratamiento que contienen dos jeringas precargadas (150 mg +100 mg) y dos agujas de seguridad adicionales.
|
- Calibre 22 x 11/2” (Eje de color gris)
- Calibre 23 x 1” (Eje de color azul)
- Jeringa precargada
- Eje
- Protector de la punta
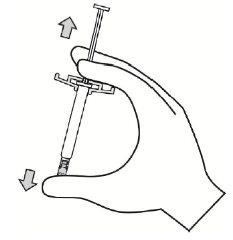
- Agite la jeringa vigorosamente como mínimo durante 10 segundos para asegurar una suspensión homogénea.
|
- Seleccione la aguja adecuada.
La primera dosis de inicio de Xeplion (150 mg) se administra el Día 1 en el músculo DELTOIDES utilizando la aguja para la inyección en el DELTOIDES. La segunda dosis de inicio de Xeplion (100 mg) se administra también en el músculo DELTOIDES una semana más tarde (Día 8) utilizando la aguja para la inyección en el DELTOIDES.
Si al paciente se le cambia de risperidona inyectable de acción prolongada a Xeplion, la primera inyección de Xeplion (rango de dosis de 25 mg a 150 mg) se puede administrar en el músculo DELTOIDES o en el GLÚTEO, utilizando la aguja apropiada para el lugar de la inyección, en el momento de la siguiente inyección programada.
Posteriormente, las inyecciones de mantenimiento mensuales se pueden administrar tanto en el músculo DELTOIDES como en el GLÚTEO utilizando la aguja adecuada para el lugar de la inyección.
En el caso de la inyección en el DELTOIDES, si el paciente pesa < 90 kg, utilice la aguja del calibre 23de 1 pulgada (25,4 mm x 0,64 mm) (aguja con eje de color azul); si el paciente pesa ? 90 kg, utilice la aguja del calibre 22de 1½ pulgadas (38,1 mm x 0,72 mm) (aguja con eje de color gris).
En el caso de la inyección en el GLÚTEO, utilice la aguja del calibre 22de 1½ pulgadas (38,1 mm x 0,72 mm) (aguja con eje de color gris).
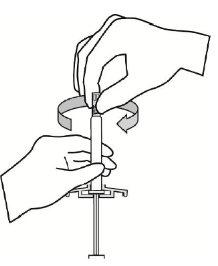
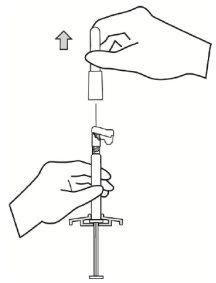
- Mientras sostiene la jeringa en posición vertical, quite el protector de goma de la punta con un movimiento de giro.
|
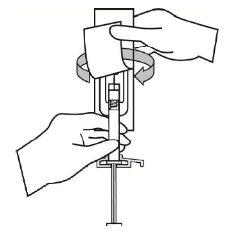
- Abra hasta la mitad la bolsa del blíster de la aguja de seguridad. Sujete la cubierta de la aguja mediante el papel de plástico de la bolsa que acaba de abrir. Acople la aguja de seguridad a la conexión luer de la jeringa con un sencillo movimiento de giro en el sentido de las agujas del reloj.
|
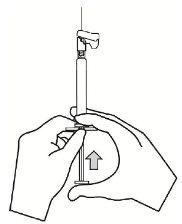
- Tire de la funda para separarla de la aguja siguiendo una línea recta. No gire la funda, dado que la aguja podría soltarse de la jeringa.
|
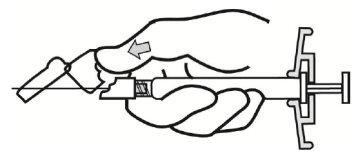
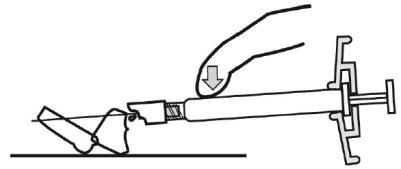
- Sitúe la jeringa con la aguja colocada en posición vertical para proceder a la eliminación del aire. Elimine el aire de la jeringa empujando el émbolo cuidadosamente hacia delante.
|
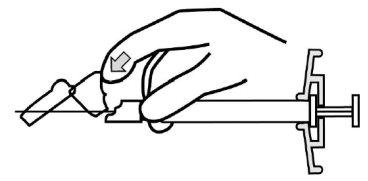
- Inyecte todo el contenido por vía intramuscular lentamente, profundamente en el músculo deltoides o glúteo seleccionado del paciente. No se debe administrar por vía intravascular osubcutánea.
- Una vez completada la inyección, utilice el pulgar u otro dedo de la mano (8a, 8b) o una superficie plana (8c) para activar el sistema de protección de la aguja. El sistema está completamente activado cuando se oye un chasquido. Deseche la jeringa con la aguja de forma adecuada.
8a
|
8b
|
8c
|
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
- País de registro
- Precio medio en farmacia262.27 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a XEPLION 150 mg SUSPENSION INYECTABLE DE LIBERACION PROLONGADAForma farmacéutica: INYECTABLE, 150 mg + 100 mgPrincipio activo: paliperidoneFabricante: Teva Pharma S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 50 mgPrincipio activo: paliperidoneFabricante: Teva Pharma S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 1000 mgPrincipio activo: paliperidoneFabricante: Janssen-Cilag International N.VRequiere receta
Médicos online para XEPLION 150 mg SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de XEPLION 150 mg SUSPENSION INYECTABLE DE LIBERACION PROLONGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes