
ULTRA-LEVURA 250 MG POLVO Y DISOLVENTE PARA SUSPENSION ORAL

Cómo usar ULTRA-LEVURA 250 MG POLVO Y DISOLVENTE PARA SUSPENSION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ultra-Levura 250 mg polvo y disolvente para suspensión oral
Saccharomyces boulardiiCNCM I-745?
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 2 días.
Contenido del prospecto:
- Qué es Ultra-Levura y para qué se utiliza.
- Qué necesita saber antes de tomar Ultra-Levura
- Cómo tomar Ultra-Levura
- Posibles efectos adversos
- Conservación de Ultra-Levura
- Contenido del envase e información adicional
1. Qué es Ultra-Levura y para qué se utiliza
Ultra-Levura es un medicamento que contiene como principio activo una levadura probiótica denominada Saccharomyces boulardii.
Está indicado para el tratamiento sintomático de la diarrea de origen inespecífico y prevención de los procesos diarreicos producidos por la administración de antibióticos en adultos y niños.
Debe consultar a un médico si empeora o si no mejora después de 2 días de tratamiento.
2. Qué necesita saber antes de tomar Ultra-Levura
No tomeUltra-Levura:
- Si es alérgico (hipersensible) al principio activo o a cualquiera de los demás componentes de Ultra-Levura, (incluidos en la sección 6).
- Si es alérgico (hipersensible) a las levaduras.
- Pacientes con catéter venoso central (Ver "Advertencias y precauciones").
- Pacientes inmunodeprimidos u hospitalizados debido a enfermedad grave o alteración/ debilitamiento del sistema inmune.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Ultra-Levura.
Tenga especial cuidado con Ultra-Levura:
- Si la diarrea va acompañada de fiebre o vómito.
- En caso que se presente sangre en las deposiciones.
- En caso de sed muy intensa o sensación de lengua seca, ya que son síntomas de deshidratación.
- No se deben abrir los frascos en los alrededores de los pacientes con catéter venoso central, para evitar cualquier colonización, especialmente las transmitidas por las manos al catéter.
Niños y adolescentes
La administración en niños menores de 2 años requerirá consejo médico.
Toma deUltra-Levuracon otros medicamentos
Comunique a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Ultra-Levura puede interaccionar con medicamentos tales como: Medicamentos antifúngicos (para tratar los hongos).
Toma deUltra-Levuracon alimentos, bebidas y alcohol
Durante el tratamiento con Ultra-Levura no se deben tomar bebidas o alimentos muy calientes (superior a 50ºC), helados o que contengan alcohol, ya que Saccharomyces boulardiicontiene células vivas.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Debe evaluarse la relación beneficio/riesgo antes de utilizarlo en embarazo y lactancia.
No se detectó ningún efecto en la fertilidad durante los estudios en animales. No existen datos clínicos, se desconoce el posible riesgo para el ser humano.
Conducción y uso de máquinas
La influencia de Ultra-Levura sobre la capacidad para conducir y utilizar máquinas es nula.
Ultra-Levura contiene lactosa, fructosa y benzoato de sodio
Este medicamento contiene lactosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene 1.520,0 mg de fructosa en cada frasco. Si su médico le ha indicado que usted (o su hijo) padecen una intolerancia a ciertos azúcares, o se les ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte con su médico antes de tomar este medicamento.
Este medicamento contiene 8,0 mg de benzoato de sodio en cada frasco. El benzoato de sodio puede aumentar el riesgo de ictericia (coloración amarillenta de la piel y los ojos) en los recién nacidos (hasta de 4 semanas de edad).
Este medicamento contiene menos de 23 mg de sodio (1mmol) por frasco; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Ultra-Levura
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
La dosis a utilizar dependerá de la evolución de los síntomas y deberá utilizarse siempre la menor dosis efectiva.
La dosis recomendada es:
Adultos y adolescentes a partir de 12 años: de 1 a 2 frascos ( 250 mg a 500 mg) al día distribuidos en dos tomas (mañana y noche).
Uso en niños
Niños a partir de 2 años: 1 frasco (250 mg) al día.
La administración en niños menores de 2 años requerirá consejo médico.
Forma de administración:
Este medicamento se toma por vía oral. Administrar preferentemente antes de las comidas.
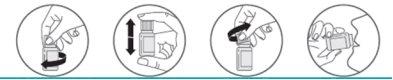
- Enroscar el tapón hasta el final, automáticamente el émbolo perfora el tabique, liberando el polvo en el disolvente contenido en el frasco.
- Agitar bien el frasco para mezclar el polvo y el disolvente.
- Desenroscar y abrir el frasco
- Beber el preparado inmediatamente.
Poblaciones especiales
Pacientes con catéter venoso central, inmunodeprimidos o en estado crítico: Este medicamento está contraindicado en estos pacientes (ver sección 2). Adicionalmente, debido al riesgo de contaminación por vía aérea, los frascos no se deben abrir en las habitaciones de estos pacientes, se debe tener especial precaución al abrirlos en las inmediaciones de los mismos y lavarse bien las manos tras la manipulación del medicamento.
Si toma másUltra-Levuradel que debe
Si ha tomado más Ultra-Levura de lo que debe, consulte inmediatamente a su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, acuda inmediatamente a su médico o llame al Servicio de Información Toxicológica, (teléfono: 91.5620420), indicando el medicamento y la cantidad ingerida.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Ultra-Levura puede producir efectos adversos, aunque no todas las personas los sufran.
El efecto adverso que más se puede producir, aunque en raras ocasiones, es flatulencia.
Los efectos adversos que se pueden producir son:
Infecciones e infestaciones
- Muy raras (<1/10.000): penetración de la levadura en la sangre (fungemia).
- Frecuencia no conocida: Infección hematológica grave (sepsis)
Alteraciones gastrointestinales
- Raras (>1/10.000 a <1/1.000): flatulencia.
- Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): estreñimiento.
Alteraciones inmunológicas
- Muy raras (<1/10.000): reacción anafiláctica con picores, urticaria, erupción cutánea, enrojecimiento de la piel e hinchazón local o general.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ultra-Levura
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar el envase en el embalaje exterior para protegerlo de la luz.
No utilice este medicamento si observa descripción de indicios visibles de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deUltra-Levura
El principio activo es: Saccharomyces boulardiiCNCM I-745: 250 mg
Los demás componentes (excipientes) son: lactosa, fructosa, aroma de frutos del bosque, ácido cítrico, sorbato de potasio, benzoato de sodio, agua purificada.
Aspecto del producto y contenido del envase
Ultra-levura es un frasco de polietileno tereftalato (PET), provisto de un tapón contenedor compuesto por una cuchilla de polipropileno (PP) y un contenedor de polietileno de baja densidad (LDPE) para el polvo (sustancia activa). El cierre del frasco es un tapón de rosca de polietileno de baja densidad (LDPE) (cápsula contra manipulación). Cada frasco contiene 8 ml de solución azucarada.
Cada envase contiene 10 ó 14 frascos.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
BIOCODEX
22 rue des Aqueducs
94250 Gentilly (Francia)
Responsable de la fabricación
BIOCODEX
1 Avenue Blaise Pascal
60000 Beauvais (Francia)
Representante local
Zambon S.A.U.
Maresme 5, Pol. Can Bernades-Subirà
08130 Sta. Perpètua de Mogoda – Barcelona (España)
Fecha de la última revisión de este prospecto:Abril 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ULTRA-LEVURA 250 MG POLVO Y DISOLVENTE PARA SUSPENSION ORALForma farmacéutica: CAPSULA, 250 mgPrincipio activo: Saccharomyces boulardiiFabricante: BiocodexNo requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 250 mgPrincipio activo: Saccharomyces boulardiiFabricante: BiocodexNo requiere recetaForma farmacéutica: CAPSULA, 50 mgPrincipio activo: Saccharomyces boulardiiFabricante: BiocodexNo requiere receta
Médicos online para ULTRA-LEVURA 250 MG POLVO Y DISOLVENTE PARA SUSPENSION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ULTRA-LEVURA 250 MG POLVO Y DISOLVENTE PARA SUSPENSION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















