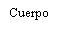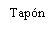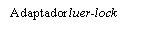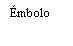TWINRIX ADULTOS, SUSPENSION INYECTABLE EN JERINGA PRECARGADA

Cómo usar TWINRIX ADULTOS, SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Twinrix Adultos, Suspensión inyectable en jeringa precargada
Vacuna (HAB) (adsorbida) antihepatitis A (inactivada) y antihepatitis B (ADNr)
Lea todo el prospecto detenidamente antes de que empiece a recibir esta vacuna,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted, y no debe dársela a otras personas.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
Contenido del prospecto
- Qué es Twinrix Adultos y para qué se utiliza
- Qué necesita saber antes de recibir Twinrix Adultos
- Cómo administrar Twinrix Adultos
- Posibles efectos adversos
- Conservación de Twinrix Adultos
- Contenido del envase e información adicional
1. Qué es Twinrix Adultos y para qué se utiliza
Twinrix Adultos es una vacuna utilizada en adultos y en adolescentes de 16 años o más para prevenir dos enfermedades: la hepatitis A y la hepatitis B. La vacuna actúa haciendo que el organismo produzca su propia protección (anticuerpos) frente a estas enfermedades.
- Hepatitis A: La hepatitis A es una enfermedad infecciosa que puede afectar al hígado. Esta enfermedad está causada por el virus de la hepatitis A. La hepatitis A se puede transmitir de persona a persona a través de los alimentos y bebidas, o nadando en aguas contaminadas por aguas residuales. Los síntomas de la hepatitis A comienzan de 3 a 6 semanas después de entrar en contacto con el virus. Éstos consisten en náuseas (malestar), fiebre y dolores. Después de varios días el blanco de los ojos y la piel se pueden volver amarillentos (ictericia). La gravedad y el tipo de síntomas pueden variar. Los niños pequeños pueden no desarrollar ictericia. La mayoría de la gente se recupera completamente pero generalmente la enfermedad es suficientemente grave como para que los pacientes estén enfermos aproximadamente durante un mes.
- Hepatitis B: La hepatitis B está causada por el virus de la hepatitis B. Causa inflamación del hígado. El virus se encuentra en los fluidos corporales tales como la sangre, el semen, las secreciones vaginales o la saliva (esputo) de las personas infectadas.
La vacunación es la mejor forma de protegerse frente a estas enfermedades. Ninguno de los componentes de la vacuna es infeccioso.
2. Qué necesita saber antes de recibir Twinrix Adultos
Twinrix Adultos no se debe administrar si:
- usted es alérgico a:
- los principios activos o a alguno de los demás componentes de esta vacuna (incluidos en la sección 6)
- la neomicina.
Los signos de una reacción alérgica pueden incluir erupción de la piel con picor, dificultad para respirar e inflamación de la cara o la lengua
- usted ha tenido anteriormente una reacción alérgica a cualquier vacuna frente a la hepatitis A y a la hepatitis B
- usted tiene una infección grave con fiebre (mayor de 38ºC). Una infección de poca importancia, como un resfriado, no debería ser un problema para la vacunación, pero dígaselo primero a su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de recibir Twinrix Adultos si:
- usted ha sufrido algún problema de salud después de la administración previa de una vacuna
- usted tiene un sistema inmune debilitado debido a una enfermedad o a un tratamiento farmacológico
- usted tiene algún problema hemorrágico o le aparecen cardenales con facilidad.
Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermero si usted se ha desmayado en anteriores ocasiones tras la administración de una inyección.
En personas obesas se ha observado una baja respuesta a la vacuna, posiblemente sin alcanzar la protección frente a la hepatitis A. También se ha observado una baja respuesta a la vacuna, posiblemente sin alcanzar la protección frente a la hepatitis B, en sujetos de edad avanzada, en hombres más que en mujeres, en fumadores, en obesos y en personas con enfermedades de larga duración, o que reciben algún tipo de tratamiento farmacológico. Su médico puede recomendarle que se someta a un análisis de sangre tras completar el ciclo de vacunación para comprobar si ha conseguido una respuesta satisfactoria. Si no es así, su médico le indicará la posibilidad de necesitar dosis adicionales.
Otros medicamentosy Twinrix Adultos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de recibir esta vacuna.
Se desconoce si Twinrix Adultos pasa a la leche materna, sin embargo no es de esperar que la vacuna cause problemas a los lactantes.
Twinrix Adultos contiene neomicina y sodio
Informe a su médico si usted ha tenido una reacción alérgica a la neomicina (antibiótico).
Esta vacuna contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo administrar Twinrix Adultos
Usted recibirá un total de tres inyecciones durante un periodo de 6 meses. Cada inyección se administrará en una visita independiente. La primera dosis se administrará en la fecha elegida. Las otras dos dosis se administrarán un mes y seis meses después de la primera dosis.
- Primera dosis: en la fecha elegida
- Segunda dosis: 1 mes después
- Tercera dosis: 6 meses después de la primera dosis
También se puede administrar un total de 3 dosis de Twinrix Adultos durante 1 mes. Esta pauta de vacunación sólo se puede administrar a adultos que necesiten una protección rápida (p.ej. viajeros). La primera dosis se administrará en la fecha elegida. Las otras 2 dosis se administrarán 7 y 21 días después de la primera dosis. Se recomienda una cuarta dosis a los 12 meses.
- Primera dosis: en la fecha elegida
- Segunda dosis: 7 días después
- Tercera dosis: 21 días después de la primera dosis
- Cuarta dosis: 12 meses después de la primera dosis
El médico le informará si son necesarias dosis adicionales y futuras dosis de recuerdo.
Como se indica en el apartado 2, es más frecuente una baja respuesta a la vacuna, posiblemente sin alcanzar la protección frente a la hepatitis B, en sujetos de edad avanzada, en hombres más que en mujeres, en fumadores, en obesos y en sujetos con enfermedades de larga duración, o que reciben algún tipo de tratamiento farmacológico. Su médico puede recomendarle que se someta a un análisis de sangre tras haber completado el ciclo de vacunación para comprobar si ha conseguido una respuesta satisfactoria. Si no es así, su médico le indicará la posibilidad de necesitar dosis adicionales.
Si se pierde una de las inyecciones previstas, hable con su médico para fijar otra visita.
Asegúrese de que termina el ciclo completo de vacunación de tres inyecciones. En caso contrario, puede no estar completamente protegido frente a las enfermedades.
El médico le administrará la inyección de Twinrix Adultos en el músculo superior del brazo.
La vacuna no debe inyectarse por vía subcutánea (profunda) o intramuscular en la nalga, ya que la protección puede ser menor.
La vacuna nunca debe inyectarse en una vena.
Si tiene cualquier otra duda sobre el uso de esta vacuna, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos que pueden ocurrir son los siguientes:
Muy frecuentes(pueden ocurrir en más de 1 de cada 10 dosis de vacuna): dolor de cabeza, dolor y enrojecimiento en el lugar de la inyección, cansancio.
Frecuentes(pueden ocurrir hasta en 1 de cada 10 dosis de vacuna): diarrea, náuseas, inflamación, cardenales o picor en el lugar de la inyección, malestar general.
Poco frecuentes(pueden ocurrir hasta en 1 de cada 100 dosis de vacuna): mareo, vómitos, dolor de estómago, dolores musculares, infección del tracto respiratorio superior, fiebre igual o mayor de 37,5°C.
Raras(pueden ocurrir hasta en 1 de cada 1.000 dosis de vacuna): inflamación de las glándulas del cuello, la axila o ingle (linfadenopatía), pérdida de sensibilidad de la piel al dolor o al tacto (hipoestesia), sensación de hormigueo (parestesia), erupción cutánea, picor, dolor articular, pérdida de apetito, presión sanguínea baja, síntomas gripales tales como fiebre, dolor de garganta, goteo nasal, tos y escalofríos.
Muy raras(pueden ocurrir hasta en 1 de cada 10.000 dosis de vacuna):
Entre los efectos adversos que ocurrieron muy raramente durante los ensayos clínicos, el uso rutinario de la vacuna o con vacunas individuales antihepatitis A y antihepatitis B se incluyen: reducción de las plaquetas, que aumenta el riesgo de sangrado o aparición de cardenales (trombocitopenia), manchas moradas o marrón rojizas visibles a través de la piel (púrpura trombocitopénica), inflamación o infección del cerebro (encefalitis), enfermedad degenerativa del cerebro (encefalopatía), inflamación de los nervios (neuritis), insensibilidad o debilidad de los brazos y piernas (neuropatía), parálisis, convulsiones o ataques, inflamación de la cara, boca o garganta (edema angioneurótico), hinchazón morada o morada rojiza de la piel (liquen plano), erupciones graves de la piel (eritema multiforme), habón urticarial, inflamación de las articulaciones, debilidad muscular, infección alrededor del cerebro que puede producir dolor de cabeza grave con rigidez de cuello y sensibilidad a la luz (meningitis), inflamación de algunos vasos sanguíneos (vasculitis), resultados anormales de las pruebas hepáticas de laboratorio, esclerosis múltiple, inflamación de la médula espinal (mielitis), párpados caídos y hundimiento de los músculos de un lado de la cara (parálisis facial), inflamación temporal de los nervios, que causa dolor, debilidad y parálisis de las extremidades y a menudo progresa al pecho y la cara (síndrome de Guillain-Barré), enfermedad de los nervios del ojo (neuritis óptica), dolor inmediato en el lugar de la inyección, escozor y sensación de ardor.
Reacciones alérgicas graves (anafilaxis, reacciones anafilactoides y reacción tipo enfermedad del suero) también pueden ocurrir muy raramente (hasta con 1 de cada 10.000 dosis de la vacuna). Algunos signos de reacciones alérgicas graves pueden ser erupciones cutáneas con picor o con ampollas, inflamación de los ojos y de la cara, dificultad para respirar o tragar, descenso repentino de la presión sanguínea y pérdida del conocimiento. Estas reacciones pueden producirse antes de abandonar la consulta del médico. En cualquier caso, si aparece cualquiera de estos síntomas debe acudir a un médico inmediatamente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Twinrix Adultos
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC).
Conservar en el embalaje original para protegerla de la luz.
No congelar. La congelación destruye la vacuna.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Twinrix Adultos
Los principios activos son:
Virus de la hepatitis A (inactivados)1,2 720 Unidades ELISA
Antígeno de superficie de la hepatitis B3,4 20 microgramos
1Producido en células diploides humanas (MRC-5)
2Adsorbido en hidróxido hidratado de aluminio 0,05 miligramos Al3+
3Producido por la tecnología del ADN recombinante en células de levadura (Saccharomyces cerevisiae)
4Adsorbido en fosfato de aluminio 0,4 miligramos Al3+
Los demás componentes de Twinrix Adultos son: cloruro de sodio y agua para preparaciones inyectables.
Aspecto deTwinrix Adultosy contenido del envase
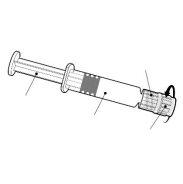
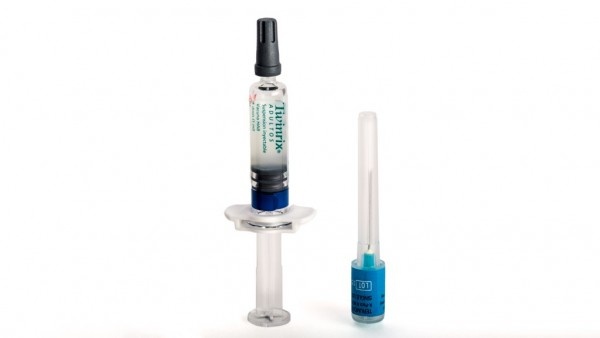
Suspensión inyectable en jeringa precargada.
Twinrix Adultos es un líquido blanco, ligeramente lechoso.
Twinrix Adultos está disponible en jeringa precargada de 1 dosis con o sin agujas separadas, tamaños de envase de 1, 10 y 25.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 | Lietuva GlaxoSmithKline Biologicals SA Tel. +370 80000334 |
???????? GlaxoSmithKline Biologicals SA ???. + 359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Magyarország GlaxoSmithKline Biologicals SA Tel.: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: + 356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλ?δα GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Tηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 970750 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 Hrvatska GlaxoSmithKline Biologicals SA Tel.: + 385 800787089 | Portugal Smith Kline & French Portuguesa - Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 România GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenija GlaxoSmithKline Biologicals SA Tel: + 386 80688869 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Biologicals SA Tel.: + 421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 774 1111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κ?προς GlaxoSmithKline Biologicals SA Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Biologicals SA Tel: + 371 80205045 | United Kingdom(Northern Ireland) GlaxoSmithKline Biologicals SA Tel: +44 (0)800 221 441 |
Fecha de la última revisión de este prospecto:04/2023
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu, y en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
Durante el almacenamiento se puede observar un depósito fino de color blanco con una capa translúcida e incolora por encima.
Se debe resuspender la vacuna antes de su uso. Una vez resuspendida, la vacuna tendrá una apariencia blanca, turbia y uniforme.
Resuspensión de la vacuna para obtener una suspensión blanca, turbia y uniforme
Se debe resuspender la vacuna siguiendo los pasos que se indican a continuación.
- Sujetar la jeringa boca arriba con la mano cerrada.
- Agitar la jeringa volteándola boca abajo y nuevamente boca arriba.
- Repetir esta acción de forma vigorosa durante, por lo menos, 15 segundos.
- Inspeccionar de nuevo la vacuna:
- Si la vacuna se muestra como una suspensión blanca, turbia y uniforme, está lista para ser usada (no debe tener una apariencia translúcida).
- Si la vacuna todavía no se muestra como una suspensión blanca, turbia y uniforme, voltearla boca abajo y nuevamente boca arriba durante, por lo menos, otros 15 segundos y a continuación inspeccionar de nuevo.
Antes de la administración, la vacuna se debe inspeccionar visualmente para observar si tiene alguna partícula extraña y/o apariencia física anormal. En caso de observar cualquiera de estas circunstancias, no administrar la vacuna.
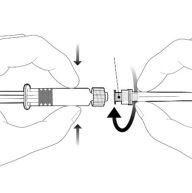
Instrucciones para la jeringa precargada tras la resuspensión
| Sostenga la jeringa por el cuerpo, no por el émbolo. Desenrosque el tapón de la jeringa girándola en sentido contrario a las agujas del reloj. | |
| Para insertar la aguja, conecte la base al adaptador luer-locky gírelo un cuarto de vuelta en el sentido de las agujas del reloj hasta que sienta que se bloquea. No saque el émbolo de la jeringa del cuerpo. Si esto ocurre, no administre la vacuna. |
Eliminación de residuos
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TWINRIX ADULTOS, SUSPENSION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: combinationsFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: combinationsFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, DescpnocidaPrincipio activo: combinationsFabricante: Glaxosmithkline BiologicalsRequiere receta
Médicos online para TWINRIX ADULTOS, SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TWINRIX ADULTOS, SUSPENSION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes