TRYDONIS 87 MICROGRAMOS/5 MICROGRAMOS/9 MICROGRAMOS SOLUCION PARA INHALACION EN ENVASE A PRESION

Cómo usar TRYDONIS 87 MICROGRAMOS/5 MICROGRAMOS/9 MICROGRAMOS SOLUCION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Trydonis 87microgramos/5microgramos/9microgramos solución para inhalación en envase a presión
dipropionato de beclometasona/fumarato de formoterol dihidrato/glicopirronio
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Trydonis y para qué se utiliza
- Qué necesita saber antes de empezar a usar Trydonis
- Cómo usar Trydonis
- Posibles efectos adversos
- Conservación de Trydonis
- Contenido del envase e información adicional
1. Qué es Trydonis y para qué se utiliza
Trydonis es un medicamento para ayudar a respirar que contiene tres principios activos:
- dipropionato de beclometasona,
- fumarato de formoterol dihidrato y
- glicopirronio.
El dipropionato de beclometasona pertenece a un grupo de medicamentos llamados corticoesteroides, que actúan reduciendo la inflamación y la irritación en los pulmones.
El formoterol y el glicopirronio son medicamentos llamados broncodilatadores de acción prolongada. Actúan de distintas formas para relajar los músculos de las vías respiratorias, ayudando a ensancharlas y permitiéndole respirar más fácilmente.
El tratamiento regular con estos tres principios activos ayuda a aliviar y prevenir síntomas tales como la dificultad para respirar, las sibilancias y la tos en los pacientes adultos con enfermedad pulmonar obstructiva crónica (EPOC). Trydonis puede reducir las exacerbaciones (empeoramientos) de los síntomas de la EPOC. La EPOC es una enfermedad grave de evolución larga en la que las vías respiratorias se bloquean y los sacos aéreos del interior de los pulmones se dañan, provocando dificultad para respirar.
2. Qué necesita saber antes de empezar a usar Trydonis
No use Trydonis:
Si es alérgico al dipropionato de beclometasona, al fumarato de formoterol dihidrato y/o al glicopirronio o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones:
Trydonis se utiliza como tratamiento de mantenimiento de la enfermedad pulmonar obstructiva. No use este medicamento para tratar una crisis repentina de dificultad para respirar o sibilancias.
Si su respiración empeora:
Si justo después de inhalar el medicamento presenta un empeoramiento de la dificultad para respirar o las sibilancias (respiraciones con un sonido silbante), interrumpa el tratamiento con el inhalador de Trydonis y utilice en seguida su inhalador “de rescate” de acción rápida. Debe ponerse en contacto con su médico de inmediato. Su médico evaluará sus síntomas y, si es necesario, puede que le cambie el tratamiento.
Ver también la sección 4. Posibles efectos adversos.
Si la enfermedad pulmonar empeora:
Si sus síntomas empeoran o son difíciles de controlar (p. ej., si está utilizando otro inhalador “de rescate” con mayor frecuencia) o si su inhalador “de rescate” no mejora sus síntomas, acuda a su médico inmediatamente. Es posible que la enfermedad pulmonar esté empeorando y su médico tenga que recetarle un tratamiento diferente.
Consulte a su médico o farmacéutico antes de empezar a usar Trydonis:
- si tiene algún problema del corazón, como angina de pecho (dolor cardiaco, dolor en el pecho), un ataque al corazón reciente (infarto de miocardio), insuficiencia cardiaca, un estrechamiento de las arterias del corazón (enfermedad coronaria), una enfermedad de las válvulas cardiacas o cualquier otra anomalía del corazón, o si tiene una enfermedad llamada miocardiopatía hipertrófica obstructiva (también conocida como MHO, una enfermedad en la que el músculo del corazón es anómalo).
- si tiene trastornos del ritmo cardiaco, como una frecuencia cardiaca irregular, una frecuencia de pulso rápida o palpitaciones o si le han dicho que su electrocardiograma (ECG) es anómalo.
- si tiene un estrechamiento de las arterias (también conocido como arteriosclerosis), si tiene la presión arterial alta o si tiene un aneurisma (un abultamiento anómalo de la pared de un vaso sanguíneo).
- si tiene una glándula tiroides hiperactiva.
- si tiene niveles bajos de potasio en la sangre (hipopotasemia). La combinación de Trydonis con ciertos medicamentos también para la EPOC o medicamentos tales como los diuréticos (medicamentos que hacen que el organismo pierda agua, para tratar una enfermedad cardiaca o la presión arterial alta) puede causar un marcado descenso de los niveles de potasio en la sangre. Por ello, es posible que su médico desee medir sus niveles de potasio en la sangre de vez en cuando.
- si padece cualquier enfermedad del hígado o los riñones.
- si tiene diabetes. Las dosis altas de formoterol pueden aumentar la glucosa en la sangre, por lo que es posible que sean necesarios algunos análisis de sangre adicionales para determinar sus niveles de azúcar en la misma cuando empiece a usar este medicamento y de vez en cuando durante el tratamiento.
- si tiene un tumor de la glándula suprarrenal (conocido como feocromocitoma).
- si se le va a administrar un anestésico. Dependiendo del tipo de anestésico, puede ser necesario interrumpir el tratamiento con Trydonis al menos 12 horas antes de la anestesia.
- si está recibiendo o ha recibido alguna vez tratamiento para la tuberculosis (TB) o si tiene una infección en el pecho.
- si tiene un problema ocular llamado glaucoma de ángulo estrecho.
- si tiene dificultades para orinar.
- si tiene una infección en la boca o la garganta.
Si se encuentra en cualquiera de las circunstancias anteriores, informe a su médico antes de empezar a usar Trydonis.
Si tiene o ha tenido cualquier problema de salud o alergia o si tiene dudas sobre si puede usar Trydonis, consulte a su médico o farmacéutico antes de empezar a usar el inhalador.
Si ya está utilizando Trydonis
Si está utilizando Trydonis o dosis altas de otros corticosteoides inhalados durante periodos de tiempo prolongados y se produce una situación de estrés (p. ej., si le han llevado al hospital tras un accidente, tiene una herida grave o antes de una operación), es posible que necesite más medicamento. En situaciones de este tipo, puede que su médico tenga que aumentarle la dosis de corticosteroides para hacer frente al estrés y puede que se los recete en forma de comprimidos o inyecciones.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Niños y adolescentes
No administre este medicamento a niños y adolescentes menores de 18 años.
Otros medicamentos y Trydonis
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto incluye los medicamentos similares a Trydonis utilizados para su enfermedad pulmonar.
Algunos medicamentos pueden aumentar los efectos de Trydonis, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
No utilice este medicamento con un medicamento betabloqueante(utilizado para tratar ciertos problemas de corazón tales como angina de pecho o para reducir la presión arterial) a menos que su médico haya escogido un betabloqueante que no afecte a su respiración. Los betabloqueantes (incluidos los betabloqueantes en colirio) pueden reducir los efectos del formoterol o hacerlos desaparecer por completo. Por otro lado, el uso de otros medicamentos beta2 agonistas (que actúan del mismo modo que el formoterol) puede aumentar los efectos del formoterol.
El uso conjunto de Trydonis con:
- medicamentos para tratar
- ritmos cardiacos anómalos (quinidina, disopiramida, procainamida),
- reacciones alérgicas (antihistamínicos),
- los síntomas de la depresión o los trastornos mentales como los inhibidores de la monoamino oxidasa (p. ej., fenelzina e isocarboxazida), antidepresivos tricíclicos (p. ej., amitriptilina e imipramina) y fenotiazinas
puede causar algunos cambios en el electrocardiograma (ECG, trazado cardiaco). También pueden aumentar el riesgo de alteraciones del ritmo cardiaco (arritmias ventriculares).
- medicamentos para tratar la enfermedad de Parkinson (levodopa), medicamentos para tratar una glándula tiroides hipoactiva (levotiroxina), medicamentos que contienen oxitocina (que causa contracciones uterinas) y el alcohol puede aumentar las posibilidades de que se produzcan efectos adversos del formoterol sobre el corazón.
- inhibidores de la monoamino oxidasa (IMAO), incluidos medicamentos con propiedades similares como la furazolidona y la procarbazina, utilizados para tratar trastornos mentales, puede causar un aumento de la presión arterial.
- medicamentos para tratar las enfermedades cardiacas (digoxina) puede causar un descenso de los niveles de potasio en la sangre. Esto puede aumentar la probabilidad de que se produzcan ritmos cardiacos anómalos.
- otros medicamentos utilizados para tratar la EPOC (teofilina, aminofilina o corticosteroides) y diuréticos también puede causar una caída de los niveles de potasio.
- algunos anestésicos puede aumentar el riesgo de que se produzcan ritmos cardiacos anómalos.
- disulfiram, un medicamento utilizado en el tratamiento de las personas con alcoholismo (problemas con el alcohol) o metronidazol, un antibiótico para tratar infecciones en su organismo puede causar efectos adversos (p. ej., náuseas, vómitos, dolor de estómago) debido a la pequeña cantidad de alcohol que contiene Trydonis.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Solo debe usar Trydonis durante el embarazo si su médico se lo aconseja. Es preferible evitar el uso de Trydonis durante el parto debido a los efectos inhibitorios del formoterol sobre las contracciones uterinas.
No debe usar Trydonis durante la lactancia. Usted y su médico deben decidir si es necesario interrumpir la lactancia o interrumpir el tratamiento tras considerar el beneficio de la lactancia para su niño y el beneficio del tratamiento para usted.
Conducción y uso de máquinas
Es improbable que Trydonis afecte a su capacidad para conducir y utilizar máquinas.
Trydonis contiene etanol
Trydonis contiene 8,856 mg de alcohol (etanol) en cada pulsación, que equivale a 17,712 mg por dosis de dos pulsaciones. La cantidad en dos pulsaciones de este medicamento es equivalente a menos de 1 ml de vino o cerveza. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible.
3. Cómo usar Trydonis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es dos dosis por la mañana y dos dosis por la noche.
Si cree que el medicamento no está resultando muy eficaz, consulte a su médico.
Si ha estado utilizando otro inhalador que contenía dipropionato de beclometasona con anterioridad, consulte a su médico, ya que la dosis eficaz de dipropionato de beclometasona de Trydonis para el tratamiento de la EPOC puede ser inferior a la de otros inhaladores.
Vía de administración
Trydonis es para uso por vía inhalatoria.
Debe inhalar el medicamento a través de la boca, lo que lleva el medicamento directamente a los pulmones.
Este medicamento se encuentra en un recipiente presurizado dentro de un inhalador de plástico con un aplicador bucal.
Trydonis está disponible en tres tamaños de envase:
- un envase que proporciona 60 dosis
- un envase que proporciona 120 dosis
- un envase que proporciona 180 dosis
Si se le ha recetado un envase que proporciona 60dosis o 120dosis
Hay un contador en la parte posterior del inhalador, que le indica cuántas dosis quedan. Cada vez que apriete el envase a presión, se liberará una dosis de medicamento y el contador restará una unidad. Evite que le caiga el inhalador ya que ello podría provocar que descontara el contador.
Si se le ha recetado un envase que proporciona 180dosis
Hay un indicador en la parte posterior del inhalador, que le indica cuántas dosis quedan. Cada vez que apriete el envase a presión, se liberará una dosis de medicamento y el contador girará un poco. El número de dosis restantes se muestra en intervalos de 20. Evite que le caiga el inhalador ya que ello podría provocar que descontara el contador.
Comprobación del inhalador
Antes de utilizar el inhalador por primera vez, debe comprobarlo del siguiente modo para asegurarse de que funciona adecuadamente.
- Dependiendo del tamaño de envase que se le haya recetado, compruebe que la lectura del contador de dosis es 61 o 121 y que la lectura del indicador de dosis es 180
- Retire el capuchón protector del aplicador bucal
- Mantenga el inhalador verticalmente con el aplicador en la parte inferior
- Dirija el aplicador bucal lejos de usted y apriete con firmeza el envase a presión para liberar una dosis
- Compruebe el contador de dosis o el indicador de dosis. Si está comprobando el inhalador por primera vez, el contador debe indicar:
60
| 120
| 180
|
|
|
|
Cómo usar el inhalador
Póngase de pie o siéntese erguido para realizar la inhalación.
IMPORTANTE: no realice los pasos 2 a 5 con demasiada rapidez.
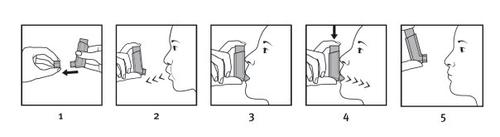
- Retire el capuchón protector del aplicador bucal y compruebe que el aplicador está limpio, es decir, que no haya restos de polvo ni suciedad.
- Espire tan lenta y profundamente como le sea posible para vaciar los pulmones.
- Mantenga el inhalador recto con el aplicador bucal hacia abajo y coloque el aplicador bucal entre los dientes sin morderlo. A continuación, sitúe los labios alrededor del aplicador bucal, con la lengua plana debajo del mismo.
- Inspire lenta y profundamente a través de la boca para llenar los pulmones de aire (esto debe llevar unos 4‑5 segundos). Justo después de comenzar a inhalar, apriete firmemente la parte superior del envase a presión para liberar una dosis.
- Aguante la respiración tanto tiempo como pueda y, finalmente, retire el inhalador de la boca y espire lentamente. No expulse el aire a través del inhalador.
- Compruebe que el contador de dosis (60/120 dosis) ha restado una unidad o que el indicador de dosis (180 dosis) ha girado un poco.
Para la segunda dosis, mantenga el inhalador en posición recta durante aproximadamente medio minuto y, a continuación, repita los pasos 2 a 5.
Si parte del gas se escapa por la parte superior del inhalador o por la comisura de los labios, significa que Trydonis no va a llegar a sus pulmones como debería. Tome otra dosis siguiendo las instrucciones empezando de nuevo por el paso 2.
Después de usarlo, vuelva a colocar el capuchón protector.
Para evitar una infección por hongos en la boca y la garganta, enjuáguese la boca o haga gárgaras con agua sin tragarla o cepíllese los dientes después de cada uso del inhalador.
Cuándo adquirir un nuevo inhalador
Debe adquirir un recambio cuando el contador o indicador muestre el número 20. Deje de usar el inhalador cuando el contador o inhalador muestre un 0, ya que todo posible medicamento restante en el inhalador puede ser insuficiente para proporcionarle una dosis completa.
Si tiene debilidad para agarrar, puede que le resulte más fácil sujetar el inhalador con ambas manos: coloque los dos dedos índices en la parte superior del inhalador y los dos pulgares en la parte inferior del inhalador.
Si le resulta difícil usar el inhalador al tiempo que comienza a inhalar, puede utilizar el dispositivo espaciador AeroChamber Plus. Consulte a su médico o farmacéutico sobre este dispositivo.
Es importante que lea el prospecto facilitado con el dispositivo espaciador AeroChamber Plus y que siga minuciosamente sus instrucciones de uso y limpieza.
Limpieza del inhalador Trydonis
Debe limpiar el inhalador una vez por semana.
- No extraiga el envase a presión del inhalador ni use agua u otros líquidos para limpiarlo.
- Retire el capuchón protector del aplicador bucal tirando de él para separarlo del inhalador.
- Limpie por dentro y por fuera el aplicador bucal y el inhalador con un paño o pañuelo de papel limpio y seco.
- Vuelva a colocar el capuchón del aplicador bucal.
Si usa más Trydonis del que debe
Es importante que tome la dosis tal como le ha indicado su médico. No supere la dosis prescrita sin consultar antes con su médico.
Si usa más Trydonis del que debe, pueden aparecer los efectos adversos descritos en la sección 4.
Informe a su médico si ha usado más Trydonis del que debe y si experimenta cualquiera de estos síntomas. Es posible que su médico quiera realizarle algunos análisis de sangre.
Si olvidó usar Trydonis
Úselo en cuanto se acuerde. Si es casi la hora de la siguiente dosis, no se administre la dosis que ha omitido, sino solo la dosis siguiente a la hora correcta. No doble la dosis.
Si interrumpe el tratamiento con Trydonis
Es importante usar Trydonis todos los días. No interrumpa el tratamiento con Trydonis ni disminuya la dosis, incluso aunque se encuentre mejor o no tenga síntomas. Si quiere hacer esto, consulte a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Existe un riesgo de empeoramiento de la dificultad para respirar y las sibilancias inmediatamente después de usar Trydonis, lo que se conoce como broncoespasmo paradójico (puede afectar hasta a 1 de cada 1.000 personas). Si esto ocurre, debe interrumpir el tratamiento con Trydonis y usar sin demora su inhalador “de rescate” de acción rápida para tratar la dificultad para respirar y las sibilancias. Debe ponerse en contacto con su médico de inmediato.
Informe a su médico inmediatamente
- si experimenta reacciones alérgicas como alergias cutáneas, ronchas, picor en la piel, erupción cutánea (puede afectar hasta 1 de cada 100 personas), enrojecimiento de la piel, hinchazón de la piel o las membranas mucosas, especialmente de los ojos, la cara, los labios y la garganta (puede afectar hasta 1 de cada 1.000 personas).
- si experimenta dolor o molestias en los ojos, visión borrosa transitoria, halos visuales o imágenes coloreadas asociadas a ojos rojos. Estos pueden ser signos de una crisis aguda de glaucoma de ángulo estrecho (pueden afectar hasta 1 de cada 10.000 personas).
Informe a su médico si nota cualquiera de los siguientes síntomas durante el uso de Trydonis, ya que pueden ser debidos a una infección pulmonar (puede afectar hasta 1 de cada 10 personas):
- fiebre o escalofríos
- aumento de la producción de moco, cambio en el color del moco
- aumento de la tos o de la dificultad para respirar
Los posibles efectos adversos se enumeran a continuación de acuerdo con su frecuencia.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- dolor de garganta
- secreción o congestión nasal y estornudos
- infecciones por hongos de la boca. Enjuagarse la boca o hacer gárgaras con agua y cepillarse los dientes inmediatamente después de la inhalación puede ayudar a prevenir estos efectos adversos
- ronquera
- dolor de cabeza
- infección del tracto urinario
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
|
|
Raros(pueden afectar hasta 1 de cada 1.000 personas):
|
|
Muy raros(pueden afectar hasta 1 de cada 10.000 personas):
- nivel bajo del número de ciertas células de la sangre llamadas plaquetas
- sensación de ahogo o de dificultad para respirar
- hinchazón de las manos y los pies
- retraso del crecimiento en niños y adolescentes
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- visión borrosa
El uso de corticoesteroides inhalados en dosis altas durante un periodo de tiempo prolongado puede causar, en casos muy raros, efectos en el organismo:
- problemas en el funcionamiento de las glándulas suprarrenales (supresión adrenal)
- disminución de la densidad mineral ósea (adelgazamiento de los huesos)
- enturbiamiento de la lente ocular (catarata)
Trydonis no contiene un corticoesteroide inhalado en dosis altas, pero es posible que su médico desee medir sus niveles de cortisol en la sangre de vez en cuando.
Los siguientes efectos adversos también pueden producirse cuando se utilizan corticoesteroides inhalados en dosis altas durante un periodo de tiempo prolongado, pero en la actualidad su frecuencia no es conocida (no puede estimarse a partir de los datos disponibles):
- depresión
- sensación de preocupación, nerviosismo, sobreexcitación o irritabilidad
Estos efectos son más probables en los niños.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Trydonis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
No congelar.
No exponer a temperaturas superiores a 50°C.
No perforar el envase a presión.
Antes de la dispensación:
Conservar en nevera (entre 2°C y 8°C).
Tras la dispensación (después de recibir este medicamento de su farmacéutico):
Envase a presión de 60 pulsaciones: | Conservar el inhalador por debajo de 25°C durante un máximo de 2 meses. |
Envase a presión de 120 (tomado de un envase individual o múltiple) y 180 pulsaciones: | Conservar el inhalador por debajo de 25°C durante un máximo de 4 meses. |
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Trydonis
Los principios activos son: dipropionato de beclometasona, fumarato de formoterol dihidrato y glicopirronio.
Cada dosis liberada (la dosis que sale del aplicador bucal) contiene 87 microgramos de dipropionato de beclometasona, 5 microgramos de fumarato de formoterol dihidrato y 9 microgramos de glicopirronio (en forma de 11 microgramos de bromuro de glicopirronio).
Cada dosis medida (la dosis que sale de la válvula) contiene 100 microgramos de dipropionato de beclometasona, 6 microgramos de fumarato de formoterol dihidrato y 10 microgramos de glicopirronio (en forma de 12,5 microgramos de bromuro de glicopirronio).
Los demás componentes son: etanol anhidro (ver sección 2), ácido clorhídrico, propelente: norflurano.
Este medicamento contiene gases fluorados de efecto invernadero.
Cada inhalador de 60 pulsaciones contiene 6,481 g de norflurano (HFC-134a) que corresponden a 0,009 toneladas de CO2 equivalente (potencial de calentamiento global PCG = 1430).
Cada inhalador de 120 pulsaciones contiene 10,37 g de norflurano (HFC-134a) que corresponden a 0,015 toneladas de CO2 equivalente (potencial de calentamiento global PCG = 1430).
Cada inhalador de 180 pulsaciones contiene 14,259 g de norflurano (HFC-134a) que corresponden a 0,02 toneladas de CO2 equivalente (potencial de calentamiento global PCG = 1430).
Aspecto del producto y contenido del envase
Trydonis es una solución para inhalación en envase a presión.
Trydonis se presenta en un envase a presión (recubierto de aluminio), con una válvula dosificadora. El envase a presión está insertado en un inhalador de plástico. Este incorpora un aplicador bucal provisto de un capuchón protector de plástico y un contador de dosis (envases con 60 y 120 dosis) o un indicador de dosis (envases con 180 dosis).
Cada envase contiene un envase a presión que proporciona 60 dosis, 120 dosis o 180 dosis. Además, existen envases múltiples que contienen 240 dosis (2 envases a presión con 120 dosis cada uno) o 360 dosis (3 envases a presión con 120 dosis cada uno).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Chiesi Farmaceutici S.p.A.
Via Palermo 26/A
43122 Parma
Italia
Responsables de la fabricación
Chiesi Farmaceutici S.p.A.
Via San Leonardo 96
43122 Parma
Italia
Chiesi SAS
Rue Faraday
ZA des Gailletrous
41260 La Chaussée Saint Victor
Francia
Chiesi Pharmaceuticals GmbH
Gonzagagasse 16/16
1010 Wien
Austria
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 | Lietuva Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
| Luxembourg/Luxemburg Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 |
Ceská republika Chiesi CZ s.r.o. Tel: + 420 261221745 | Magyarország Chiesi Hungary Kft. Tel.: + 36-1-429 1060 |
Danmark Chiesi Pharma AB Tlf: + 46 8 753 35 20 | Malta Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Deutschland Chiesi GmbH Tel: + 49 40 89724-0 | Nederland Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Eesti Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Norge Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Ελλ?δα Chiesi Hellas AEBE Τηλ: + 30 210 6179763 | Österreich Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
España Laboratorios BIAL, S.A. Tel: + 34 91 562 41 96 | Polska Chiesi Poland Sp. z.o.o. Tel.: + 48 22 620 1421 |
France Chiesi S.A.S. Tél: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Hrvatska Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | România Chiesi Romania S.R.L. Tel: + 40 212023642 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenija CHIESI SLOVENIJA, d.o.o. Tel: + 386-1-43 00 901 |
Ísland Chiesi Pharma AB Sími: +46 8 753 35 20 | Slovenská republika Chiesi Slovakia s.r.o. Tel: + 421 259300060 |
Italia Chiesi Italia S.p.A. Tel: + 39 0521 2791 | Suomi/Finland Chiesi Pharma AB Puh/Tel: +46 8 753 35 20 |
Κ?προς Chiesi Farmaceutici S.p.A. Τηλ: + 39 0521 2791 | Sverige Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvija Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Fecha de la última revisión de este prospecto: Noviembre 2024
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Precio medio en farmacia74.35 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TRYDONIS 87 MICROGRAMOS/5 MICROGRAMOS/9 MICROGRAMOS SOLUCION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 172 microgramos/5 microgramos/9 microgramosPrincipio activo: formoterol, glycopyrronium bromide and beclometasoneFabricante: Chiesi Farmaceutici S.P.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 87/5/9 microgramos/pulsaciónPrincipio activo: formoterol, glycopyrronium bromide and beclometasoneFabricante: Chiesi Farmaceutici S.P.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 88 microgramos/ 5 microgramos/ 9 microgramosPrincipio activo: formoterol, glycopyrronium bromide and beclometasoneFabricante: Chiesi Farmaceutici S.P.A.Requiere receta
Médicos online para TRYDONIS 87 MICROGRAMOS/5 MICROGRAMOS/9 MICROGRAMOS SOLUCION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TRYDONIS 87 MICROGRAMOS/5 MICROGRAMOS/9 MICROGRAMOS SOLUCION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes