TEGSEDI 284 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar TEGSEDI 284 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Tegsedi 284 mg solución inyectable en jeringa precargada
Inotersén
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas. aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tegsedi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tegsedi
- Cómo usar Tegsedi
- Posibles efectos adversos
- Conservación de Tegsedi
- Contenido del envase e información adicional
1. Qué es Tegsedi y para qué se utiliza
Tegsedi contiene el principio activo inotersén. Inotersén se utiliza para el tratamiento de adultos con amiloidosis familiar por transtiretina. La amiloidosis familiar por transtiretina es una enfermedad genética, que provoca la acumulación de pequeñas fibras de una proteína denominada transtiretina en los órganos de su cuerpo, impidiéndoles funcionar correctamente. Tegsedi se utiliza cuando la enfermedad provoca síntomas de polineuropatía (daño en los nervios).
El principio activo en Tegsedi, Inotersén, es un tipo de medicamento denominado inhibidor oligonucleótido antisentido. Actúa reduciendo la producción de transtiretina por parte del hígado, y de ese modo disminuye el riesgo de que las fibras de transtiretina se depositen en los órganos y provoquen síntomas.
2. Qué necesita saber antes de empezar a usar Tegsedi
No use Tegsedi:
- si es alérgico a inotersén o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si los análisis indican que tiene una cantidad excesivamente baja de plaquetas (las células de la sangre que se unen para ayudar a la coagulación).
- si las pruebas de función renal o de proteína en orina muestran signos de problemas renales graves.
- si tiene una reducción severa de la función hepática (insuficiencia hepática).
Advertencias y precauciones
Antes de comenzar el tratamiento con Tegsedi, su médico evaluará las células de la sangre, la función hepática, la función renal, la vitamina A y los niveles de proteínas en la orina. También podrán hacerle pruebas para asegurarse de que tiene un resultado negativo en la prueba de embarazo. A menos que su médico lo recomiende expresamente, solo se le tratará con Tegsedi si todos estos resultados están en niveles aceptables, y el resultado de su prueba de embarazo es negativo. Su médico le repetirá estos controles de manera regular durante el tratamiento. Es importante que le hagan estos análisis de sangre y de orina regularmente mientras esté administrándose Tegsedi.
Trombocitopenia
Tegsedi puede reducir las células de la sangre responsables de la coagulación (plaquetas), lo que puede dar lugar a una afección denominada trombocitopenia en cualquier momento durante el tratamiento con Tegsedi (ver sección 4). Cuando no se tienen suficientes plaquetas, como ocurre en la trombocitopenia, puede que su sangre no coagule lo suficientemente rápido como para detener las hemorragias. Esto puede provocar la formación de hematomas, además de otros problemas más graves como un sangrado excesivo o hemorragias internas. Su médico le hará análisis de sangre para controlar los niveles de plaquetas antes del tratamiento y de forma regular durante todo el tratamiento con Tegsedi. Es importante que le hagan estos análisis de sangre regularmente mientras esté recibiendo Tegsedi debido al riesgo de hemorragia grave causada por recuentos bajos de plaquetas. Si deja de usar Tegsedi, se le deberán controlar los valores sanguíneos 8 semanas después de la suspensión del medicamento.
Consultará a su médico de inmediato si presenta hematomas inexplicables o una erupción de pequeños parches rojos que aparecen en la piel (llamados petequias), sangrado por cortes en la piel que no cesa o supura, sangrado nasal o en las encías, sangre en la orina o las heces o hemorragia en la parte blanca de los ojos. Pida asistencia de inmediato si presenta rigidez en el cuello o un dolor de cabeza inusual e intenso, porque estos síntomas pueden ser causados por una hemorragia cerebral.
Glomerulonefritis/problemas renales
La glomerulonefritis es una afección de los riñones, en la que no trabajan de manera correcta debido a inflamación y daño renal. Algunos pacientes tratados con inotersén han presentado esta afección. Los síntomas de glomerulonefritis son espuma en la orina, orina de color rosa o marrón, sangre en la orina, y orinar menos de lo habitual.
Algunos pacientes tratados con inotersén también han desarrollado un deterioro en la función renal sin haber tenido glomerulonefritis.
Su médico le controlará la función renal antes del tratamiento y de forma regular durante el tratamiento con Tegsedi. Es importante que le hagan estos análisis de sangre regularmente mientras esté recibiendo Tegsedi debido al riesgo de hemorragia grave causada por recuentos bajos de plaquetas. Si deja de usar Tegsedi, se le debe controlar la función renal 8 semanas después de la suspensión del medicamento. Si desarrolla glomerulonefritis, su médico lo tratará por esta afección.
Deficiencia de vitamina A
Tegsedi puede disminuir los niveles de vitamina A (llamada también retinol) en su organismo. Su médico medirá dichos niveles, y si ya están bajos, esto se debe corregir y todos los síntomas se resolverán antes de iniciar el tratamiento con Tegsedi. Los síntomas de baja vitamina A incluyen:
- Ojos secos, visión deficiente, disminución de la visión nocturna, visión borrosa o nublada
Si tiene problemas con la vista o cualquier otro problema en los ojos mientras está usando Tegsedi, informe a su médico. Puede que su médico lo derive a un especialista en ojos para un control, en caso necesario.
Su médico le pedirá que tome un suplemento diario de vitamina A durante el tratamiento con Tegsedi.
Los niveles tanto por exceso como por deficiencia de vitamina A pueden dañar el desarrollo del niño no nacido. En consecuencia, las mujeres en edad fértil deben excluir un embarazo antes de iniciar el tratamiento con Tegsedi, y deben utilizar métodos anticonceptivos efectivos (ver sección "Embarazo y lactancia" más adelante).
Si tiene intención de quedarse embarazada, dejará de tomar inotersén, incluidos los suplementos de vitamina A, y verificar que sus niveles de vitamina A hayan vuelto a valores normales antes de intentar la concepción.
Si tiene un embarazo no planeado, dejará de tomar inotersén. Sin embargo, debido a la actividad prolongada de Tegsedi, puede que persistan sus niveles reducidos de vitamina A. Se desconoce si continuar la suplementación de 3000 UI de vitamina A por día será nocivo para su niño no nacido durante el primer trimestre de su embarazo, pero esta dosis no debe excederse. Reanudará la suplementación de vitamina A durante el segundo y el tercer trimestre de su embarazo si sus valores de vitamina A todavía no han vuelto a la normalidad, debido al mayor riesgo de deficiencia de vitamina A en el tercer trimestre.
Lesión hepática y control del hígado
Tegsedi puede causar problemas hepáticos graves. Antes de empezar a tomar inotersén, tendrá que hacerse un análisis de sangre para comprobar que su hígado funciona correctamente. También tendrá que hacerse estos análisis de sangre con regularidad mientras tome este medicamento. Es importante que se haga estos análisis de sangre con regularidad mientras tome Tegsedi.
Rechazo de trasplante hepático
Consulte a su médico antes de usar Tegsedi si ha recibido un trasplante hepático. Se han notificado casos de rechazo del trasplante hepático en pacientes tratados con Tegsedi. Su médico vigilará este aspecto en forma regular durante el tratamiento con Tegsedi.
Niños y adolescentes
Tegsedi no se debe usar en niños ni adolescentes menores de 18 años.
Otros medicamentos y Tegsedi
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Es importante que informe a su médico si ya está en tratamiento con cualquiera de los siguientes:
- Medicamentos para impedir coágulos de sangre o que disminuyen la cantidad de plaquetas en la sangre, por ejemplo, ácido acetilsalicílico, heparina, warfarina, clopidogrel, rivaroxabán y dabigatrán.
- Cualquier medicamento que pueda alterar la función renal o que pudiera dañar los riñones, por ejemplo, sulfonamidas (utilizadas como antibióticos), anilidas (utilizadas para tratar la fiebre y dolores), antagonistas de aldosterona (utilizados como diurético) y alcaloides opiáceos naturales y otros opioides (utilizados para el tratamiento del dolor).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Mujeres en edad fértil
Tegsedi reduce el nivel de vitamina A de su organismo, que es importante para un desarrollo normal del feto durante el embarazo. Se desconoce si la suplementación de vitamina A puede compensar el riesgo de deficiencia de vitamina A que podría afectar a su hijo por nacer (ver “Advertencias y precauciones” en párrafos anteriores). Si usted es una mujer en edad fértil, debe utilizar métodos anticonceptivos efectivos, y se debe excluir un embarazo antes de iniciar el tratamiento con Tegsedi.
Embarazo
No debe usar Tegsedi si está embarazada, salvo que su médico se lo aconseje en forma explícita.
Lactancia
Inotersén puede pasar a la leche materna. No se puede descartar el riesgo para el lactante. Consultará a su médico si debe interrumpir la lactancia o bien interrumpir el tratamiento con Tegsedi.
Conducción y uso de máquinas
El uso de Tegsedi no ha demostrado afectar la capacidad para conducir o usar máquinas.
Tegsedi contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 1,5 ml, por lo que está esencialmente “exento de sodio”.
3. Cómo usar Tegsedi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada de Tegsedi es una dosis de 284 mg de inotersén.
Las dosis se deben administrar una vez por semana. Todas las dosis posteriores se deben inyectar una vez por semana, el mismo día de cada semana.
Vía y forma de administración
Tegsedi es solo para inyectar bajo la piel (vía subcutánea).
Instrucciones de uso
Antes de usar la jeringa precargada, su médico le mostrará a usted o a su cuidador cómo usarla de forma correcta. Si usted o su cuidador tienen alguna duda, pregunten a su médico.
Lea las instrucciones de uso antes de comenzar a usar la jeringa precargada y cada vez que se le repita la prescripción, ya que puede haber información nueva.
Guía de las partes
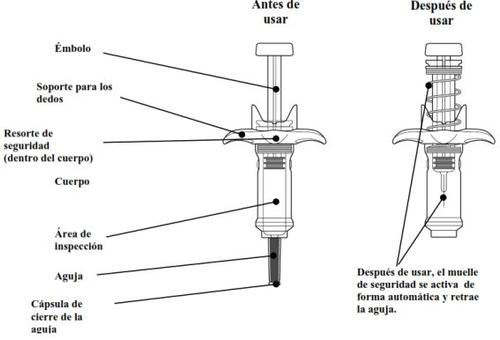
Cada jeringa precargada contiene una dosis, y es solo para un único uso.
ADVERTENCIAS | |
Noretire la cápsula de cierre de la aguja hasta que no haya llegado al Paso 6de estas instrucciones, y esté listo para inyectar Tegsedi. Nocomparta la jeringa con otra persona ni vuelva a utilizar la jeringa. Noutilizar si la jeringa precargada si cae sobre una superficie dura o si está dañada. Nocongelar la jeringa precargada. Si sucede cualquiera de las anteriores, deseche la jeringa precargada en un contenedor para objetos punzocortantes y utilice una jeringa precargada nueva. | |
PREPARACIÓN | |
| |
Noinyecte el medicamento hasta no haber reunido los elementos enumerados. | |
| |
No mueva el émbolo. | |
| |
| Mire el área de inspección para comprobar que la solución sea transparente e incolora o amarillo pálido. Es normal ver burbujas en la solución. No necesita hacer nada al respecto. Noutilizar si la solución se ve turbia, descolorida o contiene partículas. Si la solución se ve turbia, descolorida o contiene partículas, deseche la jeringa precargada en un contenedor para objetos punzocortantes (con filo), y use una jeringa precargada nueva. |
| |
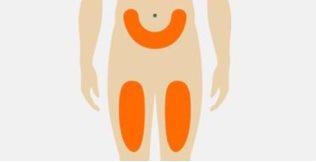
| Seleccione la zona de inyección en el abdomen (barriga) o la parte delantera del muslo. La zona de inyección puede ser también el área externa de la parte superior del brazo, si Tegsedi es administrado por un cuidador. Noinyectar en un área de 3 cm alrededor del ombligo. Noinyectar en la misma zona cada vez. Noinyectar si la piel está amoratada, dolorida, roja o dura. Noinyectar en tatuajes, cicatrices o piel dañada. Noinyectar a través de la ropa. |
| |
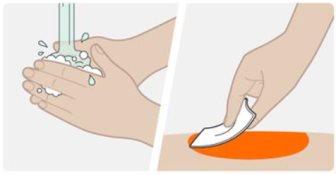
| Lávese las manos con agua y jabón. Limpie la zona de inyección con una toallita con alcohol con un movimiento circular. Deje que la piel se seque al aire. No vuelva a tocar el área antes de inyectar. |
INYECCIÓN | |
| |
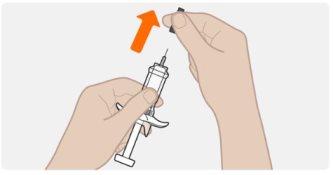
| Sostenga la jeringa precargada por el cuerpo, con la aguja apuntando hacia afuera. Retire la cápsula de cierre de la aguja tirando de él en línea recta. No lo haga girar. Puede que vea una gota de líquido en el extremo de la aguja. Esto es normal. Mantenga las manos lejos del émbolo para evitar empujar el émbolo antes de estar listo para inyectar. Noquite la cápsula de cierre de la aguja hasta justo antes de inyectar. Notire de la cápsula de cierre sosteniendo la jeringa precargada por el émbolo. Siempre sostenga el cuerpo de la jeringa. Nodeje que la aguja toque ninguna superficie. Noelimine ninguna burbuja de la jeringa precargada. Novuelva a poner el capuchón de la aguja en la jeringa precargada. |
| |
Sostenga la jeringa precargada en una mano. Sostenga la piel que rodea la zona de inyección como le ha indicado su profesional sanitario. Debe pellizcar suavemente la piel en la zona de inyección, o bien aplicar la inyección sin pellizcar la piel. Inserte lentamente la aguja en el lugar seleccionado para la inyección en un ángulo de 90° hasta que esté totalmente insertada. Nosostenga la jeringa precargada del émbolo, ni empuje contra el émbolo para insertar la aguja. | |
| |
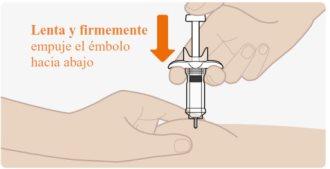
| Lenta y firmemente empuje el émbolo de manera completa hasta abajo, hasta que el medicamento haya sido inyectado. Compruebe que la aguja quede totalmente insertada en la zona de inyección mientras inyecta el medicamento. Es importante empujar el émbolo completamente hasta abajo. Puede que la jeringa precargada haga un «clic» a medida que empuja el émbolo hacia abajo. Esto es normal. Nosignifica que la inyección haya terminado. Puede que el émbolo se sienta rígido hacia el final de la inyección. Puede que necesite presionar un poco más fuerte sobre el émbolo, para asegurarse de que lo ha empujado tanto como sea posible. Nosuelte el émbolo. |
| |
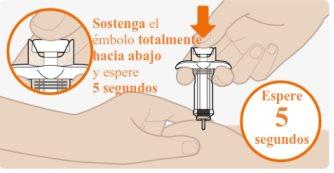
| Presione firmemente sobre el émbolo al final de la inyección. Sostenga el émbolo totalmente hacia abajo y espere 5 segundos.Si suelta el émbolo demasiado rápido, puede perder parte del medicamento. El émbolo comenzará a elevarse automáticamente, lo que significa que el émbolo fue presionado totalmente hacia abajo. Vuelva a presionar si el émbolo no empieza a elevarse automáticamente. |
| |
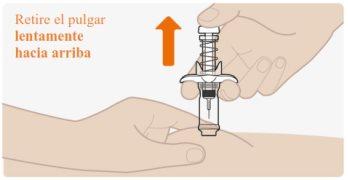
| Suelte lentamente el émbolo y deje que el muelle de seguridad retire automáticamente el émbolo hacia arriba. Ahora la aguja debe retraerse de forma segura dentro de la jeringa precargada, y el muelle del mecanismo de seguridad estará visible en la parte externa del émbolo. Cuando el émbolo se detiene, la inyección está completa. Si el émbolo no se eleva automáticamente al aflojar la presión, significa que no se activó el muelle de seguridad y usted debe volver a presionar el émbolo pero con más fuerza. Nolevante el émbolo con la mano. Levante toda la jeringa precargada en línea recta hacia arriba. Nointente volver a poner la cápsula de cierre en la aguja retraída. Nofrote el lugar de la inyección. |
ELIMINACIÓN Y CUIDADO | |
Eliminación de la jeringa precargada usada | |
| Después de su uso, poner de manera inmediata la jeringa precargada usada en un contenedor para objetos punzocortantes. No tire la jeringa precargada en la basura de su casa. |
Si usa más Tegsedi del que debe
Contacte con su médico o farmacéutico, o vaya de inmediato al servicio de urgencias de un hospital, incluso si no tiene ningún síntoma.
Si olvidó usar Tegsedi
Si olvida una dosis de Tegsedi, se administrará la dosis siguiente lo más pronto posible, salvo que la próxima dosis programada sea dentro de los dos días posteriores, en cuyo caso se debe saltar la dosis olvidada y administrarse la dosis siguiente según lo programado.
Notome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Tegsedi
No interrumpa el tratamiento con Tegsedi a menos que su médico le indique que debe hacerlo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Si presenta alguno de los siguientes efectos adversos, suspenda el uso de Tegsedi y contacte con su médico de inmediato:
- Síntomas que podrían indicar glomerulonefritis (donde sus riñones no trabajan correctamente), como espuma en la orina, orina de color rosa o marrón, sangre en la orina u orinar menos de lo habitual. La glomerulonefritis es un efecto adverso frecuente del inotersén (puede afectar hasta a 1 de cada 10 personas).
- Síntomas que podrían indicar trombocitopenia (la sangre no coagula debido a unos niveles bajos de plaquetas en sangre), como hematomas inexplicables o una erupción de pequeños parches rojos que aparecen en la piel (llamados petequias), sangrado por cortes en la piel que no cesa o supura, sangrado nasal o en las encías, sangre en la orina o las heces o hemorragia en la parte blanca de los ojos. Un nivel bajo de plaquetas en sangre es un efecto adverso muy frecuente del inotersén (puede afectar hasta a 1 de cada 10 personas).
- Síntomas que podrían indicar lesión hepática, como coloración amarillenta de los ojos o la piel, u orina oscura, posiblemente acompañada de picor en la piel, dolor en la parte superior derecha de la zona del estómago (abdomen), pérdida de apetito, sangrado o aparición de moratones con más facilidad de lo normal, o sensación de cansancio.
Pida asistencia de inmediato si presenta rigidez en el cuello o un dolor de cabeza inusual e intenso, porque estos síntomas pueden ser causados por una hemorragia cerebral.
Otros efectos adversos
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Disminución de los glóbulos rojos que puede provocar palidez en la piel y causar debilidad o dificultad para respirar (anemia)
- Dolor de cabeza
- Vómitos o náuseas (ganas de vomitar) - Aumento de la temperatura corporal
- Sensación de frío o escalofríos
- Dolor en la zona de inyección, enrojecimiento, picor o hematomas
- Hinchazón en los tobillos, pies o dedos de las manos (edema periférico)
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- Aumento en la cantidad de glóbulos blancos denominados eosinófilos (eosinofilia)
- Disminución del apetito
- Sensación de mareo o desvanecimiento, en especial al estar de pie (baja presión arterial, hipotensión)
- Cardenal
- Acumulación de sangre en los tejidos, que se puede ver como cardenal importante (hematomas)
- Picor
- Erupción
- Daño renal que puede provocar una función renal deficiente o insuficiencia renal
- Cambios en los resultados de los análisis de sangre y orina (esto puede indicar daño hepático o renal)
- Síntomas similares a los de la gripe, como temperatura alta, dolores y escalofríos
- Hinchazón o decoloración de la piel la zona de inyección
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- Reacción alérgica
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tegsedi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, la bandeja y la jeringa precargada después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC).
No congelar.
Tegsedi se puede conservar sin refrigerar hasta 6 semanas a una temperatura por debajo de 30ºC. Si no está refrigerado y no se ha utilizado en 6 semanas, el medicamento se debe desechar.
Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento si nota que el contenido está turbio o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tegsedi
- El principio activo es inotersén.
Cada ml contiene 189 mg de inotersén (como inotersén sódico). Cada jeringa precargada contiene 284 mg de inotersén (como inotersén sódico) en 1,5 ml de solución.
- Los demás componentes son agua para preparaciones inyectables, hidróxido de sodio y ácido clorhídrico (ver “Tegsedi contiene sodio” en la sección 2).
Aspecto del producto y contenido del envase
Tegsedi es una solución inyectable, transparente, de incolora a amarillo pálido (pH 7,5 – 8,8) en una jeringa precargada.
Tegsedi se presenta en tamaños de envases de 1 o 4 jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Akcea Therapeutics Ireland Ltd
St. James House,
72 Adelaide Road, Dublin 2
D02 Y017, Irlanda
Responsable de la fabricación
ABF Pharmaceutical Services GmbH
Brunnerstraße 63/18-191230 Viena
Austria
Fecha de la última revisión de este prospecto: 11/2023
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TEGSEDI 284 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 25 MGPrincipio activo: VutrisiranFabricante: Alnylam Netherlands B.V.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 300 mg/mlPrincipio activo: Oxibato sodioFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 50 mgPrincipio activo: RiluzolFabricante: Zambon S.P.A.Requiere receta
Médicos online para TEGSEDI 284 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TEGSEDI 284 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes