
TEBARAT 1 MG/ML SOLUCION PARA PULVERIZACION NASAL

Cómo usar TEBARAT 1 MG/ML SOLUCION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Tebarat 1 mg/ml solución para pulverización nasal
azelastina hidrocloruro
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Tebarat y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tebarat
- Cómo usar Tebarat
- Posibles efectos adversos
- Conservación de Tebarat
- Contenido del envase e información adicional
1. Qué es Tebarat y para qué se utiliza
Tebarat contiene azelastina hidrocloruro que pertenece a un grupo de medicamentos que recibe el nombre de antihistamínicos.
Los antihistamínicos previenen los efectos de sustancias como la histamina y otras sustancias que el cuerpo produce como parte de una reacción alérgica, las cuales originan síntomas como estornudos, moqueo, picor u obstrucción de nariz. Azelastina hidrocloruro también posee un efecto antiinflamatorio adicional.
Tebarat se utiliza para el tratamiento de los síntomas de la rinitis alérgica estacional y exacerbaciones agudas (ataques) de la rinitis alérgica perenne en adultos, adolescentes y niños a partir de los 6 años.
2. Qué necesita saber antes de empezar a usar Tebarat
No use Tebarat
Si es alérgico al principio activo (azelastina hidrocloruro) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Tebarat, si no está seguro de si sus trastornos están causados por una alergia.
Niños y adolescentes
Tebarat no se recomienda en niños menores de 6 años.
Otros medicamentos y Tebarat
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se han estudiado interacciones específicas.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Debido a la vía de administración nasal y a las bajas dosis administradas, puede esperarse una exposición sistémica (general) mínima. Sin embargo, así como ocurre con todos los medicamentos, se deben tomar precauciones durante su uso en embarazo y lactancia.
Conducción y uso de máquinas
No se han descrito efectos sobre la capacidad de conducción o uso de máquinas por el uso de Tebarat.
3. Cómo usar Tebarat
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada (adultos y niños mayores de 6 años) es una pulverización (0,14 ml) de Tebarat en cada fosa nasal, dos veces al día. Esto corresponde a una dosis diaria de 0,56 mg de azelastina hidrocloruro.
Uso en personas de edad avanzada: no se han realizado estudios específicos.
Instrucciones de uso


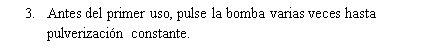
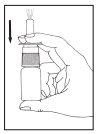

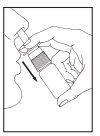
Si usa más Tebarat del que debe
Si usted ha pulverizado demasiado Tebarat en sus fosas nasales, consulte a su médico o farmacéutico.
Con la vía de administración nasal no se prevén reacciones de sobredosificación.
Los estudios en animales muestran que las dosis tóxicas pueden producir síntomas sobre el Sistema Nervioso Central, como ej. excitación, temblor, convulsiones. Si esto ocurriese en humanos, se iniciará un tratamiento sintomático y de soporte. Si la sobredosificación es reciente se recomienda un lavado gástrico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Tebarat
No tome una dosis doble para compensar las dosis olvidadas.
Si olvidó usar su medicamento, úselo tan pronto como lo recuerde y póngase la siguiente dosis 12 horas después, en caso de ser necesario.
Si interrumpe el tratamiento con Tebarat
No interrumpa el tratamiento bruscamente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Estos efectos incluyen:
- Frecuentes (pueden afectar hasta 1 de cada 10 personas): tras la administración puede aparecer un sabor amargo debido con frecuencia a una incorrecta aplicación, por ejemplo, con la cabeza demasiado inclinada hacia atrás durante la administración.
- Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): puede producirse una irritación de la mucosa nasal con síntomas como escozor, picor, estornudos, epistaxis (pequeñas hemorragias nasales).
- Raros (pueden afectar hasta 1 de cada 1.000 personas): náuseas.
- Muy raros (pueden afectar hasta 1 de cada 10.000 personas): hipersensibilidad, mareo, fatiga, debilidad, erupción, prurito, urticaria.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano http://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tebarat
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del frasco y en la caja después de CAD. La fecha de caducidad es el ultimo día del mes que se indica.
No utilice Tebarat después de 60 días de la apertura del frasco.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tebarat
- El principio activo es azelastina hidrocloruro 1 mg por ml.
- Los demás components son hipromelosa, edetato disódico, ácido cítrico, hidrogenofosfato de sodio heptahidrato, cloruro de sodio y agua purificada.
Aspecto del producto y contenido del envase
Tebarat es una solución incolora y transparente que se presenta en frascos de vidrio ámbar de 20 ml provistos de una válvula dosificadora, que contiene una solución para pulverización nasal.
Titular de la autorización de comercialización
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat
Barcelona – España
Responsable de la fabricación
Pharmaloop, S.L.
C/ Bolivia, 15 – Polig Industrial Azque
28806 Alcalá de Henares
Madrid – España
Laboratorios Salvat, S.A.
C/ Gall, 30-36
08950 – Esplugues de Llobregat (Barcelona)
España
Estemedicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
España Tebarat 1 mg/ml solución para pulverización nasal
Italia Tebarat
Portugal Tebarat 1 mg/ml solução para pulverização nasal
Fecha de la última revisión de este prospecto:
Agosto 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia11.33 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TEBARAT 1 MG/ML SOLUCION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 0,1 g azelastina hidrocloruro/100 mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere receta
Médicos online para TEBARAT 1 MG/ML SOLUCION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TEBARAT 1 MG/ML SOLUCION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











