
SYCREST 10 MG COMPRIMIDOS SUBLINGUALES

Cómo usar SYCREST 10 MG COMPRIMIDOS SUBLINGUALES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Sycrest 5mg comprimidos sublinguales
Sycrest 10mg comprimidos sublinguales
asenapina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Sycrest y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Sycrest
- Cómo tomar Sycrest
- Posibles efectos adversos
- Conservación de Sycrest
- Contenido del envase e información adicional
1. Qué es Sycrest y para qué se utiliza
Sycrest contiene el principio activo asenapina. Este medicamento pertenece al grupo de medicamentos denominados antipsicóticos. Sycrest se utiliza para tratar los episodios maníacos de moderados a graves asociados con el trastorno bipolar I en adultos. Los medicamentos antipsicóticos afectan las sustancias químicas que permiten la comunicación entre las células nerviosas (neurotransmisores). Las enfermedades que afectan al cerebro, como el trastorno bipolar I, pueden deberse al desequilibrio de ciertas sustancias químicas del cerebro, como dopamina y serotonina, y estos desequilibrios pueden causar algunos de los síntomas que está experimentando. No se conoce exactamente cómo actúa este medicamento, sin embargo se cree que ajusta el equilibrio de estas sustancias químicas.
Los episodios maníacos asociados con el trastorno bipolar I son una enfermedad con síntomas tales como sentirse bien, tener una energía exagerada, necesidad de dormir mucho menos de lo habitual, hablar muy deprisa con multitud de ideas y a veces gran irritabilidad.
2. Qué necesita saber antes de empezar a tomar Sycrest
No tome Sycrest
Si es alérgico a asenapina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Sycrest.
Sycrest no se ha estudiado en pacientes de edad avanzada con demencia. Sin embargo, estos pacientes que están tratándose con otros tipos de medicamentos similares, pueden tener un mayor riesgo de accidente cerebrovascular (ictus) o muerte. Sycrest no ha sido aprobado para el tratamiento de pacientes de edad avanzada con demencia y no está recomendado para su uso en este grupo particular de pacientes.
Sycrest puede causar una bajada de la tensión arterial. En las primeras etapas del tratamiento, algunas personas pueden desmayarse, sobre todo si se incorporan después de estar tumbados o sentados. Normalmente desaparece por sí mismo, pero si no es así, dígaselo a su médico, ya que puede necesitar un ajuste en su dosis.
Asenapina puede causar somnolencia, caídas repentinas de la tensión arterial cuando se levanta, mareo y cambios en su capacidad para moverse y en el equilibrio, que pueden conllevar a caídas y, por consiguiente, fracturas u otras lesiones traumáticas. Se debe evaluar a los pacientes con riesgo de caída antes de recetar asenapina.
Informe a su médico inmediatamente si experimenta
- movimientos rítmicos involuntarios de la lengua, boca y cara. Puede ser necesario suspender el tratamiento con Sycrest.
- fiebre, agarrotamiento muscular intenso, sudoración o disminución del nivel de consciencia (un trastorno llamado "síndrome neuroléptico maligno"). Puede necesitar tratamiento médico inmediato.
Antes de tomar Sycrest, compruebe con su médico o farmacéutico:
- si alguna vez le han diagnosticado una enfermedad cuyos síntomas son alta temperatura corporal y rigidez muscular (también conocido como síndrome neuroléptico maligno).
- si alguna vez ha experimentado movimientos anormales de la lengua o de la cara (discinesia tardía).
Debe estar atento ya que ambas enfermedades pueden ser causadas por este tipo de medicamento.
- si padece una enfermedad del corazón o está en tratamiento de una enfermedad del corazón que pueda hacerle propenso a tener la tensión arterial baja
- si padece diabetes o es propenso a la diabetes
- si padece enfermedad de Parkinson o demencia
- si tiene epilepsia (convulsiones)
- si experimenta dificultad al tragar (disfagia)
- si tiene problemas graves del hígado. Si los tiene, no debe tomar Sycrest
- si tiene problema de control de la temperatura corporal
- si tiene pensamientos de suicidio
- si tiene niveles anormalmente altos de prolactina en la sangre (hiperprolactinemia)
Asegúrese de contarle a su médico si se encuentra en cualquiera de estas circunstancias para que él/ella pueda ajustar su dosis o hacerle seguimiento durante algún tiempo. También consulte a su médico inmediatamente si desarrolla cualquiera de estas dolencias o empeoran mientras está usando Sycrest.
Niños y adolescentes
No se recomienda el uso de Sycrest en pacientes menores de 18 años.
Otros medicamentos y Sycrest
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Algunos medicamentos pueden reducir o aumentar el efecto de Sycrest.
Si está tomando otros medicamentos, Sycrest debe tomarse el último.
Debe contarle a su médico si está tomando medicamentos antidepresivos (en concreto fluvoxamina, paroxetina o fluoxetina), ya que puede ser necesario cambiar su dosis de Sycrest o su dosis de antidepresivo.
Debe contarle a su médico si está tomando medicamentos para la enfermedad de Parkinson (como levodopa), ya que este medicamento puede hacerlos menos efectivos.
Sycrest actúa principalmente en el cerebro, por lo que puede haber interferencia con otros medicamentos (o con alcohol) que también actúan en el cerebro debido a una suma de efectos en la función cerebral.
Sycrest puede disminuir la tensión arterial por lo que se debe tener cuidado cuando Sycrest se toma junto con otros medicamentos que disminuyen la tensión arterial.
Toma de Sycrest con alimentos, bebidas y alcohol
No comer ni beber durante 10 minutos después de tomar este medicamento.
Debe evitar beber alcohol cuando está tomando este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No tome Sycrest mientras esté embarazada, a menos que su médico se lo indique. Si está tomando este medicamento y se queda embarazada o planea quedarse embarazada, consulte a su médico lo antes posible para saber si puede continuar tomando Sycrest.
Se pueden producir los siguientes síntomas en bebés recién nacidos, de madres que han sido tratadas con Sycrest en el último trimestre de embarazo (últimos tres meses de su embarazo): temblor, rigidez y/o debilidad muscular, somnolencia, agitación, problemas al respirar, y dificultad en la alimentación. Si su bebé desarrolla cualquiera de estos síntomas se debe poner en contacto con su médico.
No dé el pecho a su bebé mientras esté tomando Sycrest.
Conducción y uso de máquinas
Sycrest puede producir somnolencia o sedación. Por lo tanto, asegúrese de que su concentración y estado de alerta no están afectados antes de conducir o utilizar máquinas.
3. Cómo tomar Sycrest
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es un comprimido sublingual de 5 ó 10 mg dos veces al día. Una dosis debe tomarse por la mañana y otra dosis debe tomarse por la noche.
Instrucciones de uso
Sycrest es para uso sublingual.
No se recomienda Sycrest si no es capaz de tomar el comprimido como se describe a continuación. Si no es capaz de tomar este medicamento como se describe a continuación, el tratamiento puede que no sea efectivo para usted.
- No sacar el comprimido sublingual del blíster hasta el momento de tomarlo.
- Tener las manos secas cuando toque el comprimido.
- No presionar el comprimido sobre el blíster. No cortar ni romper el blíster.
- Despegar la lengüeta coloreada (Figura 1).
- Sacar con cuidado el comprimido (Figura 2). No aplastar el comprimido.
- Para asegurar una óptima absorción, colocar el comprimido bajo la lengua y esperar hasta que se disuelva completamente (Figura 3). El comprimido se disolverá en la saliva en unos segundos.
- No tragar ni masticar el comprimido.
- No comer ni beber durante 10 minutos después de tomar el comprimido.
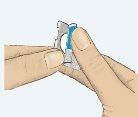
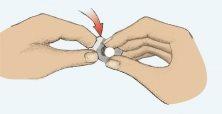
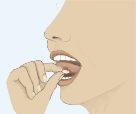
Figura 1 Figura 2 Figura 3
Si toma más Sycrest del que debe
Si toma demasiado Sycrest, contacte con un médico inmediatamente. Lleve el medicamento con usted. En caso de sobredosis puede sentirse somnoliento o cansado, tener movimientos corporales anormales, problemas al estar de pie o caminando, sentirse mareado debido a una bajada de la tensión arterial y sentirse agitado y confundido.
Si olvidó tomar Sycrest
No tome una dosis doble para compensar las dosis olvidadas. Si olvidó tomar una dosis, tome su siguiente dosis como siempre. Si olvidó tomar dos o más dosis, consulte con su médico o farmacéutico.
Si interrumpe el tratamiento con Sycrest
Si interrumpe el tratamiento con Sycrest, perderá los efectos de este medicamento. No debe interrumpir el tratamiento con este medicamento, a menos que su médico se lo indique, ya que sus síntomas pueden reaparecer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado efectos adversos graves con este medicamento. Solicite atención médica de inmediato si tiene cualquiera de los siguientes síntomas:
- reacciones alérgicas (suelen implicar una combinación de efectos tales como dificultad para respirar o tragar, hinchazón de la cara, labios, lengua o de la garganta, erupción cutánea, picazón y aumento de la frecuencia cardíaca.)
- aumento repentino de la temperatura corporal, con sudoración, pulso acelerado, rigidez muscular grave, confusión y tensión arterial variable, que puede conllevar al coma
- convulsiones, ataques o crisis
- desmayo
- las caídas se pueden producir como resultado de uno o más efectos adversos como: somnolencia, caídas repentinas de la tensión arterial cuando se levanta, mareo y cambios en su capacidad para moverse y en el equilibrio.
Comunique a su médico de inmediato si tiene:
- signos de aumento de los niveles de azúcar en sangre como sed, hambre u orina excesiva, debilidad o aparición de un empeoramiento de la diabetes
- movimientos serpenteantes de la lengua, u otros movimientos incontrolados de la lengua, boca, mejillas, mandíbula que puede progresar a los brazos y a las piernas
Otros efectos adversos comunicados con este medicamento son:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- ansiedad
- somnolencia
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- aumento de peso
- aumento del apetito
- contracciones musculares lentas o sostenidas
- inquietud
- contracciones musculares involuntarias
- movimientos lentos, temblor
- sedación
- mareo
- náuseas
- cambio en el gusto
- sensación de entumecimiento en la lengua o en la boca
- saliva aumentada (babeo)
- tirantez muscular
- fatiga
- aumento del nivel de proteínas del hígado
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- movimientos anormales de los músculos: conjunto de síntomas conocidos como síntomas extrapiramidales, que pueden incluir uno o más de los siguientes: movimientos anormales de los músculos, lengua o mandíbula, contracciones musculares lentas o sostenidas, espasmos musculares, temblor (agitación), movimientos anormales de los ojos, contracciones musculares involuntarias, movimientos lentos o inquietud
- sensación desagradable en las piernas (también conocido como síndrome de la pierna inquieta)
- dificultades al hablar
- latido anormalmente lento o rápido
- bloqueo central del corazón
- electrocardiograma anormal (prolongación del intervalo QT)
- bajada de la tensión arterial al ponerse de pie
- tensión arterial baja
- cosquilleo en la lengua o en la boca
- lengua hinchada o dolorosa
- dificultad al tragar
- úlceras, dolor, enrojecimiento, hinchazón y ampollas dentro de la boca
- disfunción sexual
- ausencia de periodos menstruales regulares
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas)
- alteración de los niveles de células blancas de la sangre (leucocitos)
- dificultad para enfocar la vista
- coágulos sanguíneos en vasos sanguíneos hacia los pulmones, que causan dolor de pecho y dificultad para respirar
- enfermedad muscular que se manifiesta con dolores inexplicables
- aumento del pecho en el hombre
- extravasación de leche o fluidos de la mama
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Sycrest
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el blíster y en el envase. La fecha de caducidad es el último día del mes que se indica.
Conservar este medicamento en el embalaje original para protegerlo de la luz y de la humedad.
Este medicamento no requiere ninguna temperatura especial de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Sycrest
- El principio activo es asenapina.
- Cada comprimido sublingual de Sycrest 5 mg contiene 5 mg de asenapina.
- Cada comprimido sublingual de Sycrest 10 mg contiene 10 mg de asenapina.
- La cantidad exacta se muestra en su envase de Sycrest.
- Los demás componentes son gelatina y manitol (E‑421).
Aspecto del producto y contenido del envase
Los comprimidos sublinguales de 5 mg son comprimidos redondos, de blanco a blanquecino, marcados con "5" en una de las caras.
Los comprimidos sublinguales de 10 mg son comprimidos redondos, de blanco a blanquecino, marcados con "10" en una de las caras.
Los comprimidos sublinguales se suministran en blísters despegables que contienen 10 comprimidos cada uno. Los envases contienen 20, 60 ó 100 comprimidos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
N.V. Organon
Kloosterstraat 6
NL-5349 AB Oss
Países Bajos
Responsable de la fabricación
Organon Heist bv
Industriepark 30
2220 Heist‑op‑den‑Berg, Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Organon Belgium Tél/Tel: 0080066550123 (+32 2 2418100) | Lietuva Organon Pharma B.V. Lithuania atstovybe Tel.: +370 52041693 |
???????? ??????? (?.?.) ?.?. - ???? ???????? ???.: +359 2 806 3030 | Luxembourg/Luxemburg Organon Belgium Tél/Tel: 0080066550123 (+32 2 2418100) |
Ceská republika Organon Czech Republic s.r.o. Tel: +420 233 010 300 | Magyarország Organon Hungary Kft. Tel.: +36 1 766 1963 |
Danmark Organon Denmark ApS Tlf: +45 4484 6800 | Malta Organon Pharma B.V., Cyprus branch Tel: +356 2277 8116 |
Deutschland Organon Healthcare GmbHTel.: 0800 3384 726 (+49 (0) 89 2040022 10) [email protected] | Nederland N.V. Organon Tel: 00800 66550123 (+32 2 2418100) |
Eesti Organon Pharma B.V. Estonian RO Tel: +372 66 61 300 | Norge Organon Norway AS Tlf: +47 24 14 56 60 |
Ελλ?δα BIANEΞ Α.Ε. Τηλ: +30 210 80091 11 | Österreich Organon Healthcare GmbH Tel: 0800 3384 726 (+49 (0) 89 2040022 10) |
España Organon Salud, S.L. Tel: +34 91 591 12 79 | Polska Organon Polska Sp. z o.o. Tel.: +48 22 105 50 01 |
France Organon France Tél: +33 (0) 1 57 77 32 00 | Portugal Organon Portugal, Sociedade Unipessoal Lda. Tel: +351?218705500 |
Hrvatska Organon Pharma d.o.o. Tel: +385 1 638 4530 | România Organon Biosciences S.R.L. Tel: +40 21 527 29 90 |
Ireland Organon Pharma (Ireland) Limited Tel: +353 15828260 | Slovenija Organon Pharma B.V., Oss, podružnica Ljubljana Tel: +386 1 300 10 80 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Organon Slovakia s. r. o. Tel: +421 2 44 88 98 88 |
Italia Organon Italia S.r.l. Tel: +39 06 90259059 | Suomi/Finland Organon Finland Oy Puh/Tel: +358 (0) 29 170 3520 |
Κ?προς Organon Pharma B.V., Cyprus branch Τηλ: +357 22866730 | Sverige Organon Sweden AB Tel: +46 8 502 597 00 |
Latvija Arvalsts komersanta “Organon Pharma B.V.” parstavnieciba Tel: +371 66968876 | United Kingdom (Northern Ireland) Organon Pharma (UK) Limited Tel: +44 (0) 208 159 3593 |
Fecha de la última revisión de este prospecto:{mes/AAAA}.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Precio medio en farmacia156.32 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SYCREST 10 MG COMPRIMIDOS SUBLINGUALESForma farmacéutica: COMPRIMIDO SUBLINGUAL, 5 mgPrincipio activo: asenapineFabricante: Organon N.V.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 9,1 MGPrincipio activo: LoxapinaFabricante: Ferrer Internacional S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 9,1 mgPrincipio activo: LoxapinaFabricante: Ferrer Internacional S.A.Requiere receta
Médicos online para SYCREST 10 MG COMPRIMIDOS SUBLINGUALES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SYCREST 10 MG COMPRIMIDOS SUBLINGUALES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











