
SOGROYA 15 MG/1,5 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar SOGROYA 15 MG/1,5 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Sogroya 15mg/1,5ml solución inyectable en pluma precargada
somapacitán
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Sogroya y para qué se utiliza
- Qué necesita saber antes de empezar a usar Sogroya
- Cómo usar Sogroya
- Posibles efectos adversos
- Conservación de Sogroya
- Contenido del envase e información adicional
1. Qué es Sogroya y para qué se utiliza
Sogroya contiene el principio activo somapacitán: una versión de larga duración de la hormona del crecimiento natural que produce el organismo con una sustitución de un aminoácido. La hormona del crecimiento regula la composición de grasa, músculos y huesos en adultos.
El principio activo de Sogroya se fabrica con “tecnología de ADN recombinante”, a partir de células que han recibido un gen (ADN) que hace que produzcan la hormona del crecimiento. En Sogroya, se ha añadido una pequeña cadena lateral a la hormona del crecimiento que une Sogroya a la proteína (albúmina) que se encuentra presente de forma natural en la sangre para ralentizar su eliminación del organismo, lo que permite administrar el medicamento con menor frecuencia.
Sogroya se utiliza para tratar el retraso en el crecimiento en niños a partir de 3 años de edad y adolescentes si tienen una nula o muy baja producción de hormona de crecimiento (deficiencia de la hormona de crecimiento) y adultos con deficiencia de la hormona del crecimiento.
Su médico evaluará, en función de su respuesta a Sogroya, si debe continuar el tratamiento con Sogroya un año después de empezar con este medicamento.
2. Qué necesita saber antes de empezar a usar Sogroya
No use Sogroya
- si usted o un menor a su cargo es alérgico a somapacitán o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si usted o un menor a su cargo tiene un tumor benigno o maligno en desarrollo. Debe haber terminado el tratamiento antitumoral antes de empezar el tratamiento con Sogroya. Si el tumor crece debe dejar de recibir Sogroya.
- si usted o un menor a su cargo se ha sometido recientemente a una cirugía a corazón abierto o abdominal o ha sufrido traumatismo accidental múltiple o presenta problemas respiratorios graves o afecciones similares.
- en niños y adolescentes que han dejado de crecer debido al cierre de sus placas de crecimiento (epífisis cerradas) lo cual significa que su médico de referencia le ha indicado a usted o al menor a su cargo, que sus huesos han dejado de crecer.
En caso de duda, consulte a su médico, farmacéutico o enfermero.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de usar Sogroya si:
- usted o un menor a su cargo alguna vez ha tenido algún tipo de tumor
- usted o un menor a su cargo tiene un nivel de azúcar en sangre alto (hiperglucemia), ya que es posible que tenga que comprobar regularmente su nivel de azúcar en sangre y tenga que ajustar la dosis de su medicamento para la diabetes
- usted o un menor a su cargo está recibiendo una terapia de sustitución con corticosteroides porque su organismo no produce la cantidad suficiente (insuficiencia adrenocortical). Hable con su médico, ya que podría necesitar ajustes regulares
- usted o un menor a su cargo experimenta dolor de cabeza intenso, problemas de visión, náuseas o vómitos, ya que pueden ser síntomas de un aumento de la presión cerebral (hipertensión intracraneal benigna) ya que podría necesitar interrumpir el tratamiento
- usted o un menor a su cargo tiene problemas de tiroides, debe comprobar sus hormonas tiroideas periódicamente y puede necesitar ajustar la dosis de hormonas tiroideas
- es una mujer que toma anticonceptivos orales o una terapia de sustitución hormonal con estrógenos, puede necesitar un aumento de la dosis de Sogroya. Si deja de tomar estrógenos orales puede necesitar una disminución de la dosis de somapacitán. Su médico puede recomendarle que cambie la forma de administración del estrógeno (p. ej., transdérmica, vaginal) o usar otro método anticonceptivo
- usted o un menor a su cargo se encuentra gravemente enfermo (por ejemplo, complicaciones de una cirugía a corazón abierto, cirugía abdominal, traumatismo accidental, fallo respiratorio agudo o enfermedades similares). Si se ha sometido o se va a someter a una operación mayor, o va a asistir al hospital por alguna de las razones anteriores, informe a su médico y recuerde a otros médicos que puedan tratarle de que está en tratamiento con la hormona del crecimiento
- usted o un menor a su cargo sufre un dolor de estómago intenso durante el tratamiento con Sogroya, ya que esto podría ser un síntoma de inflamación del pancreas observado en tratamientos con otros medicamentos de hormona de crecimiento
- usted o un menor a su cargo sufren dolor persistente de cadera o rodilla al caminar, o si usted o un menor a su cargo comienzan a cojear durante el tratamiento con hormona del crecimiento. Estos podrían ser síntomas de una enfermedad que afecta el fémur donde se inserta en la cadera (epífisis femoral superior) y que ocurre con mayor frecuencia en niños de crecimiento rápido y niños con trastornos endocrinos, incluida la deficiencia de la hormona del crecimiento. Informe al médico si sufre un dolor persistente en cualquier articulación.
Cambios en la piel en la zona de inyección
La zona de inyección de Sogroya se debe rotar para prevenir cambios en el tejido adiposo bajo la piel, como engrosamiento de la piel, encogimiento de la piel o bultos bajo la piel. Cambie la zona de inyección en su cuerpo cada semana.
Anticuerpos
No se espera que desarrolle anticuerpos contra somapacitán. Sin embargo, solo en muy raras ocasiones el niño puede desarrollar anticuerpos. Si su tratamiento con Sogroya no funciona, su médico tendrá que comprobar si ha desarrollado anticuerpos a somapacitán.
Otros medicamentos y Sogroya
Informe a su médico o farmacéutico si usted o un menor a su cargo está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
En concreto, informe a su médico si usted o un menor a su cargo está tomando o ha tomado recientemente alguno de los siguientes medicamentos.
El motivo es que su médico puede tener que ajustar las dosis de sus medicamentos:
- Corticosteroides como hidrocortisona, dexametasona y prednisolona
- Estrógenos como parte de anticoncepción oral o una terapia de sustitución hormonal con estrógenos
- Hormonas sexuales masculinas (andrógenos) como testosterona
- Gonadotropinas (hormonas estimuladoras de gónadas, como la hormona luteinizante y la hormona foliculoestimulante), que estimulan la producción de hormonas sexuales
- Insulina u otros medicamentos para la diabetes
- Medicamentos para las hormonas tiroideas como levotiroxina
- Medicamentos para tratar la epilepsia o las convulsiones (crisis epilépticas) como carbamazepina
- Ciclosporinas (inmunosupresor), un medicamento que anula la respuesta del sistema inmunitario.
Embarazo
- Si puede quedarse embarazada, no debe utilizar Sogroya a menos que esté utilizando un método anticonceptivo fiable. Esto se debe a que no se sabe si podría dañar al feto. Si se queda embarazada durante el tratamiento con Sogroya, informe a su médico inmediatamente. Si desea quedarse embarazada, hable con su médico, ya que es posible que tenga que dejar de utilizar el medicamento.
Lactancia
- No se conoce si Sogroya se excreta en la leche materna. Informe a su médico si está en periodo de lactancia o tiene intención de hacerlo. Su médico le ayudará a decidir si deja la lactancia o deja de administrarse Sogroya, considerando el beneficio de la lactancia para el bebé y el beneficio de Sogroya para la madre.
Conducción y uso de máquinas
Sogroya no afecta a la capacidad para conducir y utilizar máquinas.
Contenido de sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Sogroya
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Sogroya se inyecta bajo la piel (inyección subcutánea) con una pluma precargada. Puede administrarse la inyección usted mismo. Su médico o enfermero le indicará la dosis adecuada y le mostrará cómo inyectarse cuando usted o un menor a su cargo empiece el tratamiento.
Cuándo usar Sogroya
- Usted o un menor a su cargo debe usar Sogroya una vez a la semana, el mismo día de la semana a ser posible.
- Puede administrarse la inyección a cualquier hora del día.
Si usted o un menor a su cargo cambia de otro tratamiento semanal de hormona de crecimiento a Sogroya, se recomienda continuar inyectándose el mismo día de la semana.
Si usted o un menor a su cargo cambia de un tratamiento diario de hormona de crecimiento a Sogroya, elija el día preferido para la administración semanal e inyecte la última dosis del tratamiento diario el día anterior (o al menos 8 horas antes) a la inyección de la primera dosis de Sogroya.
Cualquier cambio desde otro tipo o marca de hormona de crecimiento debe ser realizado por su médico.
Si no es posible que usted o un menor a su cargo se inyecte Sogroya su día habitual de la semana, puede administrar Sogroya hasta 2 días antes o 3 días después del día programado. La semana siguiente puede inyectarse la proxima dosis de la manera habitual.
En caso necesario, puede cambiar el día de la inyección semanal de Sogroya, siempre que hayan transcurrido al menos 4 días desde la última inyección. Una vez seleccionado el nuevo día de administración, debe continuar inyectándose la dosis el mismo día cada semana.
Durante cuánto tiempo necesitará tratamiento
Puede necesitar Sogroya siempre y cuando su cuerpo no produzca suficiente hormona de crecimiento
- Si usted o un menor a su cargo están utilizando Sogroya para el retraso del crecimiento, seguirán utilizando Sogroya hasta que dejen de crecer
- Si usted o un menor a su cargo sigue padeciendo déficit de hormona de crecimiento después de dejar de crecer, puede que necesite continuar su tratamiento con Sogroya hasta la edad adulta
No interrumpa su tratamiento con Sogroya sin hablar previamente con su médico.
Cuánto usar
Niños y adolescentes
La dosis para niños y adolescentes depende del peso corporal.
La dosis recomendada de Sogroya es 0,16 mg por kg de peso corporal administrada una vez a la semana.
Adultos
La dosis inicial habitual es 1,5 mg una vez a la semana si es la primera vez que recibe el tratamiento con la hormona del crecimiento. La dosis inicial habitual es 2 mg una vez a la semana si ha recibido previamente un tratamiento diario con la hormona del crecimiento (somatropina).
Si es una mujer que toma estrógenos orales (anticonceptivos o un tratamiento de sustitución) puede necesitar una dosis mayor de somapacitán. Si tiene más de 60 años, puede que necesite una dosis menor. Consulte la Tabla 1 más abajo.
Puede que su médico aumente o disminuya la dosis de forma escalonada y regular hasta que encuentre la dosis adecuada según sus necesidades individuales y los efectos adversos experimentados.
- No utilice más de 8 mg una vez a la semana.
- No cambie la dosis a menos que su médico se lo indique.
Tabla 1 Dosis recomendada
Población adulta con deficiencia de la hormona del crecimiento | Dosis inicial recomendada |
No ha recibido previamente tratamiento diario con la hormona del crecimiento | |
Es ≥18 y <60 años Es una mujer en tratamiento con estrógenos orales independientemente de la edad Tiene 60 años en adelante | 1,5 mg/semana 2 mg/semana 1 mg/semana |
Ha recibido previamente tratamiento diario con la hormona del crecimiento | |
Es ≥18 y <60 años Es una mujer en tratamiento con estrógenos orales independientemente de la edad Tiene 60 años en adelante | 2 mg/semana 4 mg/semana 1,5 mg/semana |
Tras haber alcanzado la dosis correcta, su médico evaluará su tratamiento cada 6 a 12 meses. Puede que tenga que revisar su índice de masa corporal y extraer muestras de sangre.
Cómo usar Sogroya
Su médico o enfermero le indicarán cómo inyectarse Sogroya bajo la piel.
Los mejores lugares para inyectarse son:
- la parte frontal del muslo
- la parte frontal de la cintura (abdomen)
- los glúteos
- la parte superior de los brazos.
Cambie la zona de inyección en su cuerpo cada semana.
Las instrucciones detalladas sobre cómo inyectar Sogroya, instrucciones de uso, se incluyen al final de este prospecto.
Si usa más Sogroya del que debe
Si usted o un menor a su cargo utiliza accidentalmente más Sogroya del que debe, consulte a su médico, ya que puede que tenga que comprobar sus niveles de azúcar en sangre.
Si olvidó usar Sogroya
Si usted o un menor a su cargo olvidó inyectarse una dosis:
- y han pasado 3 días o menos desde que debería haber usado Sogroya, adminístreselo tan pronto como se acuerde. Después, inyéctese la siguiente dosis el día habitual de inyección
- y han pasado más de 3 días desde que debería haber usado Sogroya, sáltese la dosis olvidada. Después, inyéctese la siguiente dosis como de costumbre, el día siguiente programado.
No se inyecte una dosis adicional o aumente la dosis para compensar la dosis olvidada.
Si interrumpe el tratamiento con Sogroya
No interrumpa el tratamiento con Sogroya sin consultar con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos observados en niños y adolescentes
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza.
Frecuentes (pueden afectar hasta a 1 de cada 10 personas)
- Hinchazón en manos y pies debido a una acumulación de líquidos bajo la piel (edema periférico)
- Las glándulas suprarrenales no producen suficientes hormonas esteroideas (insuficiencia adrenocortical)
- Disminución de la hormona tiroidea (hipotiroidismo)
- Enrojecimiento y dolor en el lugar de la inyección (reacciones en la zona de inyección)
- Dolor articular (artralgia)
- Dolor en brazos o piernas (dolor en las extremidades)
- Nivel de azúcar en sangre alto (hiperglucemia)
- Sensación de estar muy cansado (fatiga).
Efectos adversos observados en adultos
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza.
Frecuentes (pueden afectar hasta a 1 de cada 10 personas)
- Las glándulas suprarrenales no producen suficientes hormonas esteroideas (insuficiencia adrenocortical)
- Disminución de la hormona tiroidea (hipotiroidismo)
- Nivel de azúcar en sangre alto (hiperglucemia)
- Sensación de “hormigueo”, sobre todo en los dedos (parestesia)
- Sarpullido
- Urticaria
- Dolor articular (artralgia), dolor muscular (mialgia), rigidez muscular
- Hinchazón en manos y pies debido a una acumulación de líquidos bajo la piel (edema periférico)
- Sensación de mucho cansancio o debilidad (fatiga o astenia)
- Enrojecimiento y dolor en el lugar de la inyección (reacciones en la zona de inyección).
Poco frecuentes (pueden afectar hasta a 1 de cada 100 personas)
- Engrosamiento de la piel en el lugar donde se inyecta el medicamento (lipohipertrofia)
- Sensación de entumecimiento y hormigueo en las manos (síndrome del túnel carpiano)
- Picor (prurito)
- Rigidez articular.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Sogroya
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja de la pluma después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar. Mantener alejado del componente de enfriamiento de la nevera.
Después del primer uso
Usar en un plazo de 6 semanas después del primer uso. Conservar en nevera (entre 2 ºC y 8 ºC).
Antes y después de primer uso
Si no puede refrigerarlo (por ejemplo durante un viaje), se puede conservar Sogroya provisionalmente a temperaturas menores de 30 ºC durante un máximo total de 72 horas (3 días). Vuelva a conservar Sogroya en nevera de nuevo tras mantenerlo a esta temperatura. Si lo ha conservado fuera de nevera y luego ha vuelto a almacenarlo en nevera, el tiempo total fuera de refrigeración no debe de exceder los 3 días, haga un seguimiento minucioso de ello. Deseche la pluma de Sogroya si se ha mantenido a 30 ºC durante más de 72 horas o a más 30 ºC en cualquier periodo de tiempo.
Registre el tiempo fuera de nevera:_____________
Conserve Sogroya en el embalaje exterior con el capuchón de la pluma puesto para protegerlo de la luz.
Retire siempre la aguja después de cada inyección y conserve la pluma sin la aguja puesta.
No utilice este medicamento si observa que la solución no es transparente o ligeramente opalescente, de incolora a ligeramente amarilla y no está libre de partículas visibles.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Sogroya
- El principio activo es somapacitán. Un ml de solución contiene 10 mg de somapacitán. Cada pluma precargada contiene 15 mg de somapacitán en 1,5 ml de solución.
- Los demás componentes son: histidina, manitol, poloxamer 188, fenol, agua para preparaciones inyectables, ácido clorhídrico (para ajuste del pH), hidróxido de sodio (para ajuste del pH). Consulte también la sección 2 “Qué necesita saber antes de empezar a usar Sogroya” para más información sobre el sodio.
Aspecto del producto y contenido del envase
Sogroya es un líquido inyectable en una pluma precargada con un aspecto transparente o ligeramente opalescente, incoloro o ligeramente amarillo y libre de partículas visibles.
Sogroya 15 mg/1,5 ml solución inyectable en pluma precargada con pulsador de color rojo rubí está disponible en los siguientes tamaños de envase: un envase que contiene 1 pluma precargada o un envase múltiple que contiene 5 envases, cada uno con 1 pluma precargada.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Dinamarca
Fecha de la última revisión de este prospecto:06/2025
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
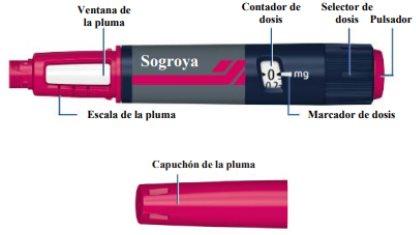
Instrucciones de uso Vista general de la pluma Sogroya 15mg/1,5ml
Aguja (ejemplo)
| |
Cómo usar la pluma Sogroya Se deben seguir 5pasos durante la inyección de Sogroya: Paso 1. Preparación de la pluma Sogroya..........................................................................................89 Paso 2. Comprobación del flujo con cada pluma nueva.....................................................................90 Paso 3. Selección de la dosis..............................................................................................................91 Paso 4. Inyección de la dosis..............................................................................................................92 Paso 5. Después de la inyección........................................................................................................93 Para más información sobre la pluma, ver las secciones:Compruebe cuánto Sogroya queda, Cómo cuidar la pluma, Información importante. Lea atentamente el prospecto y estas instrucciones antes de utilizar la pluma precargada Sogroya. Preste especial atención a estas notas porque son importantes para el uso seguro de la pluma. Información adicional Sogroya contiene 15 mg de somapacitán y se puede utilizar para inyectar dosis desde 0,10 mg a 8 mg en intervalos de 0,1 mg. Sogroya solo se puede utilizar bajo la piel (vía subcutánea). Las agujas no están incluidas y se deberán obtener por separado. La pluma precargada Sogroya está diseñada para utilizarse con agujas desechables de 4 mm a 8 mm de longitud y de 30 G a 32 G de grosor. Nocomparta la pluma Sogroya ni las agujas con otra persona. Puede infectar a esa persona o contraer una infección. No utilice la pluma sin haber recibido la formación adecuada de su médico o enfermero.Asegúrese de que siente la confianza necesaria para inyectarse usted mismo con la pluma antes de comenzar el tratamiento. Si es usted invidente o tiene visión reducida y no puede leer el contador de dosis de la pluma, no utilice esta pluma sin ayuda. Pida ayuda a una persona sin problemas de visión y formada en el uso de la pluma. | |
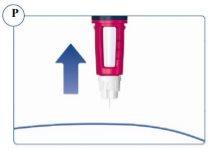
Paso 1. Preparación de la pluma Sogroya | |
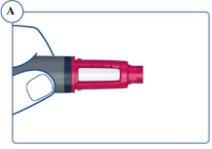
Asegúrese de que utiliza la pluma correcta. Especialmente si utiliza más de un tipo de medicamento inyectable. El uso de un medicamento equivocado puede ser perjudicial para su salud. |
|
|
|
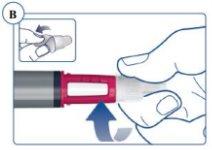
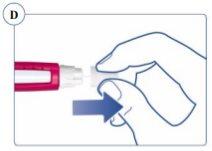
La aguja está protegida por dos capuchones. Debe retirar ambos capuchones. Si olvida retirar alguno, no se inyectará ningún medicamento. Ver figuras C y D. |
|
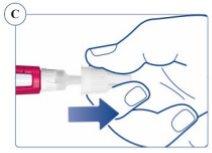
Puede aparecer una gota de Sogroya en la punta de la aguja. Esto es normal, pero a pesar de ello debe comprobar el flujo con cada pluma nueva. Ver Paso 2. |
|
Utilice siempre una aguja nueva para cada inyección.Así se reduce el riesgo de contaminación, infección, pérdida de Sogroya y que las agujas se atasquen y se administren dosificaciones inexactas. Nunca utilice una aguja doblada o dañada. | |
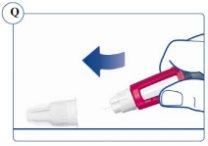
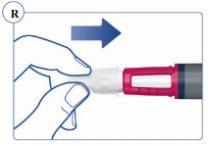
Paso 2. Comprobación del flujo con cada pluma nueva | |
Si su pluma ya está en uso, vaya al Paso 3.
|
|
|
|
|
|
Si no aparece Sogroya, repita el Paso 2 un máximo de 6 veces. Si sigue sin aparecer ninguna gota de Sogroya, cambie la aguja una vez como se describe en el Paso 5 y repita los Pasos 1 y 2 de nuevo. |
|
Si no aparece Sogroya cuando comprueba el flujo, puede que la aguja esté atascada o dañada. No utilice la pluma si sigue sin aparecer Sogroya tras cambiar la aguja. Puede que la pluma esté defectuosa. | |
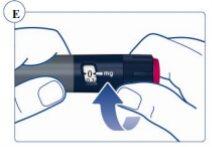
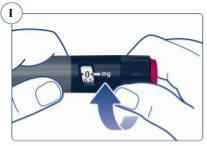
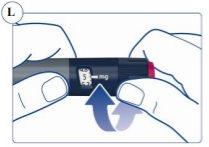
Paso 3. Selección de la dosis | |
Una vez ha seleccionado la dosis, continúe con el Paso 4. Si no queda suficiente Sogroyapara seleccionar una dosis completa, consulte Compruebe cuánto Sogroya queda. |
|
El contador de dosis muestra la dosis en mg. Ver figuras J y K. Utilice siempre el marcador de dosis para seleccionar la dosis exacta. No cuente los clics de la pluma. No utilice la escala de la pluma(ver Vista general de la pluma Sogroya) para medir la cantidad de hormona del crecimiento que se va a inyectar. Solo el marcador de dosis indicará el número exacto de mg. |
|
Si selecciona una dosis incorrecta, puede girar el selector de dosis en el sentido de las agujas del reloj o a la inversa para corregir la dosis. Ver figura L. Los clics de la pluma suenan y se sienten de forma diferente cuando se gira el selector de dosis en sentido de las agujas del reloj o a la inversa o si por error se pasa de la cantidad de mg restantes. |
|
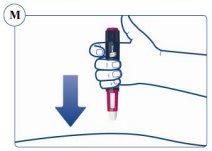
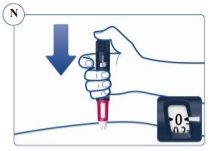
Paso 4. Inyección de la dosis | |
Compruebe que puede ver el contador de dosis. No lo tape con los dedos.Esto podría bloquear la inyección. Recuerde cambiar la zona de inyección cada semana. |
|
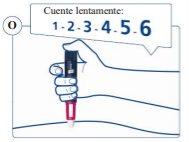
Continúe presionando el pulsador con la aguja en la piel. |
|
la piel y cuente lentamente hasta 6para asegurarse de que administra la dosis completa (ver figura O). |
|
Si no aparece el “0” en el contador de dosis después de presionar continuamente el pulsador, puede que la aguja o la pluma estén bloqueadas o dañadas y no haya recibido nada de Sogroya, incluso aunque el contador de dosis se haya movido desde la dosis original que fijó. Retire la aguja como se describe en el Paso 5 y repita los Pasos 1 a 4. | |
Puede aparecer una gota de Sogroya en la punta de la aguja después de la inyección. Esto es normal y no afecta a la dosis. |
|
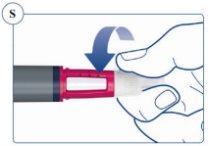
Paso 5. Después de la inyección | |
|
|
|
|
Deseche siempre la aguja después de cada inyección. Cuando la pluma esté vacía, extraiga y deseche la aguja como aparece anteriormente y tire la pluma por separado, siguiendo las instrucciones de su médico, enfermero, farmacéutico o las autoridades locales. El capuchón de la pluma y el envase vacío pueden tirarse a la basura. |
|
Para conservar la pluma, consulte Conservación de Sogroyaen este folleto. |
|
No intente volver a colocar el capuchón interior de la aguja. Podría pincharse con la aguja. Retire siempre la aguja de la pluma inmediatamente después de cada inyección. Así se reduce el riesgo de contaminación, infección, pérdida de Sogroya y que las agujas se atasquen y, por tanto, se administren dosificaciones inexactas. | |
Compruebe cuánto Sogroya queda | |
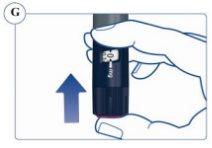
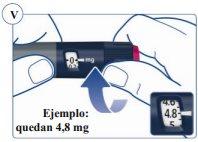
La escala de la pluma muestra la cantidad aproximada de Sogroya que queda en la pluma. Ver figura U. |
|
Para saber cuánto Sogroya queda, utilice el contador de dosis: Gire el selector de dosis en el sentido de las agujas del reloj hasta que el contador de dosis se detenga. Puede seleccionar una dosis máxima de 8 mg. Si muestra “8” quedan al menos 8 mg en la pluma. Si el contador de dosis se detiene en “4,8”, solo quedan 4,8 mg en la pluma. Ver figura V. |
|
¿Qué ocurre si necesito una dosis mayor a la que queda en la pluma? | No es posible seleccionar una dosis mayor a la cantidad de mg que queda en la pluma. Si necesita más Sogroya del que queda en la pluma, puede usar una pluma nueva o dividir la dosis entre la pluma en uso y la nueva. Solo puede dividir la dosis si su médico o enfermero se lo ha recomendado y ha recibido la formación pertinente.Utilice una calculadora para planificar las dosis siguiendo las indicaciones de su médico o enfermero. Tenga mucho cuidado de hacer el cálculo correctamente, ya que puede provocar un error de medicación.En caso de duda sobre cómo dividir la dosis en dos plumas, seleccione e inyéctese la dosis que necesita con una pluma nueva. |
Cómo cuidar la pluma | |
¿Cómo debo cuidar la pluma? | Tenga cuidado de no dejar caer la pluma ni golpearla contra superficies duras. No exponga la pluma al polvo, la suciedad, líquidos o la luz directa. No intente rellenar la pluma, está precargada y se debe desechar cuando esté vacía. |
¿Qué ocurre si se cae la pluma? | Si la pluma se cae o cree que no funciona correctamente, inserte una nueva aguja desechable y compruebe el flujo antes de la inyección, ver Pasos 1 y 2. Si la pluma se ha caído, compruebe el cartucho, si se ha agrietado, no utilice la pluma. |
¿Cómo limpio la pluma? | No lave, ponga a remojo ni lubrique la pluma. Se puede limpiar con un detergente suave con un paño humedecido. |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SOGROYA 15 MG/1,5 ML SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 10 MGPrincipio activo: somapacitanFabricante: Novo Nordisk A/SRequiere recetaForma farmacéutica: INYECTABLE, 5 MGPrincipio activo: somapacitanFabricante: Novo Nordisk A/SRequiere recetaForma farmacéutica: INYECTABLE, 12 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere receta
Médicos online para SOGROYA 15 MG/1,5 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SOGROYA 15 MG/1,5 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











 Información importante
Información importante





