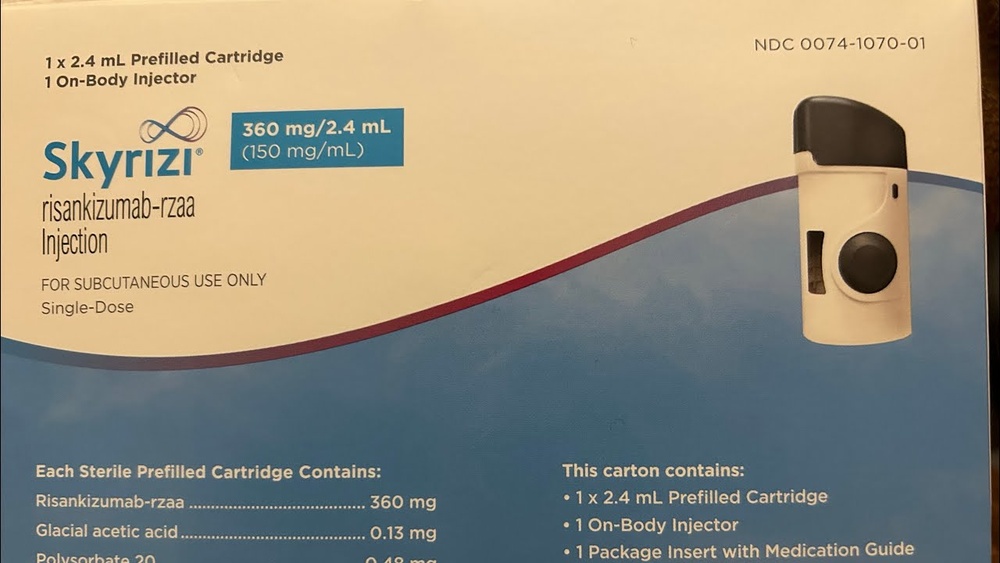
SKYRIZI 180 MG SOLUCION INYECTABLE EN CARTUCHO

Cómo usar SKYRIZI 180 MG SOLUCION INYECTABLE EN CARTUCHO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Skyrizi 180mg solución inyectable en cartucho
Skyrizi 360mg solución inyectable en cartucho
risankizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Skyrizi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Skyrizi
- Cómo usa Skyrizi
- Posibles efectos adversos
- Conservación de Skyrizi
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es Skyrizi y para qué se utiliza
Skyrizi contiene el principio activo risankizumab.
Skyrizi se usa para tratar a pacientes adultos con:
- enfermedad de Crohn de moderada a grave
- colitis ulcerosa de moderada a grave
Cómo funciona Skyrizi
Este medicamento actúa bloqueando una proteína del organismo llamada “IL-23” que provoca inflamación.
Enfermedad de Crohn
La enfermedad de Crohn es una enfermedad inflamatoria del aparato digestivo. Si tiene enfermedad de Crohn activa, es posible que primero le administren otros medicamentos. Si estos medicamentos no funcionan lo suficientemente bien, se le administrará Skyrizi para el tratamiento de su enfermedad de Crohn.
Colitis ulcerosa
La colitis ulcerosa es una enfermedad inflamatoria del intestino grueso. Si tiene colitis ulcerosa activa, es posible que primero le administren otros medicamentos. Si estos medicamentos no funcionan lo suficientemente bien o si usted no puede tomarlos, se le administrará Skyrizi para tratar su colitis ulcerosa.
Skyrizi reduce la inflamación y, por ello, puede ayudar a reducir los signos y síntomas de su enfermedad.
2. Qué necesita saber antes de empezar a usar Skyrizi
No use Skyrizi
- si es alérgico a risankizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una infección que su médico considera importante, por ejemplo, tuberculosis activa.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Skyrizi y durante su tratamiento:
- si padece una infección actualmente o si tiene una infección que reaparece.
- si padece tuberculosis (TB).
- si ha recibido recientemente o tiene previsto recibir una vacuna. Ciertas vacunas no deben administrarse durante el tratamiento con Skyrizi.
Es importante conservar una copia del número de lote de Skyrizi.
Cada vez que reciba un nuevo envase de Skyrizi, anote la fecha y el número de lote (que aparece en el envase después de “Lot”) y guarde esta información en un lugar seguro.
Reacciones alérgicas
Consulte a su médico o solicite atención médica de inmediato si advierte algún signo de reacción alérgica mientras recibe Skyrizi, por ejemplo:
- dificultad para respirar o tragar
- hinchazón de la cara, los labios, la lengua o la garganta
- picor intenso en la piel, con una erupción roja o bultos
Niños y adolescentes
Skyrizi no está recomendado en niños y adolescentes menores de 18 años de edad, ya que no se ha estudiado el uso de Skyrizi en este grupo de edad.
Otros medicamentos y Skyrizi
Informe a su médico, farmacéutico o enfermero:
- si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- si se ha vacunado recientemente o tiene previsto vacunarse. Ciertas vacunas no deben administrarse durante el tratamiento con Skyrizi.
En caso de duda, consulte a su médico, farmacéutico o enfermero antes de usar Skyrizi y durante su tratamiento.
Embarazo, anticoncepción y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Es necesario que lo haga porque no se sabe cómo afectará este medicamento al bebé.
Si es una mujer que puede quedarse embarazada, debe utilizar anticonceptivos mientras esté en tratamiento con este medicamento y durante un mínimo de 21 semanas después de su última dosis de Skyrizi.
Si está en periodo de lactancia o tiene intención de amamantar a un bebé, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Es poco probable que Skyrizi afecte a su capacidad para conducir y usar máquinas.
Skyrizi contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por cartucho; esto es, esencialmente “exento de sodio”.
3. Cómo usar Skyrizi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Este medicamento se administra mediante una inyección bajo la piel (llamada “inyección subcutánea”).
Cuánto Skyrizi utilizar
Comenzará el tratamiento con Skyrizi a una dosis inicial que le administrará su médico o enfermero mediante goteo en el brazo (perfusión intravenosa).
Dosis iniciales
¿Cuánto? | ¿Cuándo? | |
Enfermedad de Crohn | 600 mg | Cuando le indique su médico |
600 mg | 4 semanas después de la 1.ª dosis | |
600 mg | 4 semanas después de la 2.ª dosis |
Colitis ulcerosa | ¿Cuánto? | ¿Cuándo? |
1 200 mg | Cuando le indique su médico | |
1 200 mg | 4 semanas después de la 1.ª dosis | |
1 200 mg | 4 semanas después de la 2.ª dosis |
Posteriormente, recibirá Skyrizi mediante una inyección bajo la piel.
Dosis de mantenimiento
Enfermedad de Crohn | ¿Cuánto? | ¿Cuándo? |
1.ª dosis de mantenimiento | 360 mg | 4 semanas después de la última dosis inicial (en la semana 12) |
Dosis siguientes | 360 mg | Cada 8 semanas, comenzando después de la 1.ª dosis de mantenimiento |
Colitis ulcerosa | ¿Cuánto? | ¿Cuándo? |
1.ª dosis de mantenimiento | 180 mg o 360 mg | 4 semanas después de la última dosis inicial (en la semana 12) |
Dosis siguientes | 180 mg o 360 mg | Cada 8 semanas, comenzando después de la 1.ª dosis de mantenimiento |
Usted y su médico, farmacéutico o enfermero decidirán si se podría inyectar este medicamento usted mismo. No debe inyectarse este medicamento usted mismo a menos que su médico, farmacéutico o enfermero le hayan enseñado cómo hacerlo. También es posible que le administre la inyección un cuidador que haya aprendido a hacerlo.
Lea la sección7 “Instrucciones de uso” al final de este prospecto antes de ponerse la inyección de Skyrizi.
Si usa más Skyrizi del que debe
Si ha utilizado más Skyrizi del que debe o se ha administrado la dosis antes de lo prescrito, consulte a su médico.
Si olvidó usar Skyrizi
Si olvida administrarse Skyrizi, se debe inyectar una dosis en cuanto se acuerde. En caso de duda, consulte a su médico.
Si interrumpe el tratamiento con Skyrizi
No deje de utilizar Skyrizi sin hablar antes con su médico. Si interrumpe el tratamiento, sus síntomas pueden reaparecer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Consulte a su médico o solicite atención médica de inmediato si tiene algún síntoma de infección grave, por ejemplo:
- fiebre, síntomas pseudogripales, sudores nocturnos
- sensación de cansancio o dificultad para respirar, tos persistente
- calor, enrojecimiento y dolor en la piel o una erupción cutánea dolorosa con ampollas
Su médico decidirá si puede seguir utilizando Skyrizi.
Otros efectos adversos
Informe a su médico, farmacéutico o enfermero si nota alguno de los siguientes efectos adversos
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- infecciones de las vías respiratorias altas con síntomas como dolor de garganta y congestión nasal.
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- sensación de cansancio
- infección de hongos en la piel
- reacciones en el lugar de inyección (como enrojecimiento o dolor)
- prurito
- dolor de cabeza
- erupción
- eczema
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- pequeños bultos rojos en la piel
- ronchas (urticaria)
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Skyrizi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del cartucho y en la caja exterior después de EXP.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Si es necesario, también puede conservar el cartucho fuera de la nevera (a una temperatura máxima de 25 °C) durante un máximo de 24 horas.
Conservar el cartucho en el embalaje original para protegerlo de la luz.
No utilice este medicamento si el líquido está turbio o contiene escamas o partículas grandes.
Cada inyector corporal con cartucho es para un solo uso.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Skyrizi
El principio activo es risankizumab.
Skyrizi 180 mg solución inyectable en cartucho
- Cada cartucho contiene 180 mg de risankizumab en 1,2 ml de solución.
- Los demás componentes son acetato de sodio trihidrato, ácido acético, trehalosa dihidrato, polisorbato 20 y agua para preparaciones inyectables.
Skyrizi 360 mg solución inyectable en cartucho
- Cada cartucho contiene 360 mg de risankizumab en 2,4 ml de solución.
- Los demás componentes son acetato de sodio trihidrato, ácido acético, trehalosa dihidrato, polisorbato 20 y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Skyrizi es un líquido transparente de incoloro a color amarillo contenido en un cartucho. El líquido puede contener partículas diminutas transparentes o blancas.
Cada envase contiene 1cartucho y 1inyector corporal.
Titular de la autorización de comercialización y responsable de la fabricación
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien AbbVie SA Tél/Tel: +32 10 477811 | Lietuva AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tél/Tel: +32 10 477811 |
Ceská republika AbbVie s.r.o. Tel: +420 233 098 111 | Magyarország AbbVie Kft. Tel: +36 1 455 8600 |
Danmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta V.J.Salomone Pharma Limited Tel: +356 22983201 |
Deutschland AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (gebührenfrei) Tel: +49 (0) 611 / 1720-0 | Nederland AbbVie B.V. Tel: +31 (0)88 322 2843 |
Eesti AbbVie OÜ Tel: +372 623 1011 | Norge AbbVie AS Tlf: +47 67 81 80 00 |
Ελλ?δα AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Österreich AbbVie GmbH Tel: +43 1 20589-0 |
España AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Polska AbbVie Sp. z o.o. Tel: +48 22 372 78 00 |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Hrvatska AbbVie d.o.o. Tel: +385 (0)1 5625 501 | România AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenija AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Ísland Vistor hf. Tel: +354 535 7000 | Slovenská republika AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italia AbbVie S.r.l. Tel: +39 06 928921 | Suomi/Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 |
Κ?προς Lifepharma (Z.A.M.) Ltd Τηλ: +357 22 34 74 40 | Sverige AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvija AbbVie SIA Tel: +371 67605000 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
La información detallada y actualizada sobre este producto está disponible a continuación o en el embalaje exterior escaneando el código QR a través de un smartphone. La misma información también está disponible en el siguiente sitio web: www.skyrizi.eu
Código QR a incluir
Para solicitar una copia de este prospecto en
- Instrucciones de uso
Lea toda la sección7 antes de utilizar Skyrizi
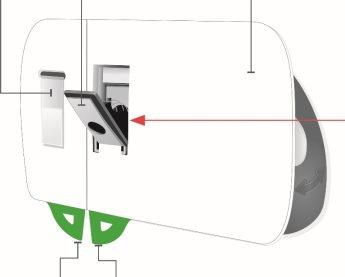
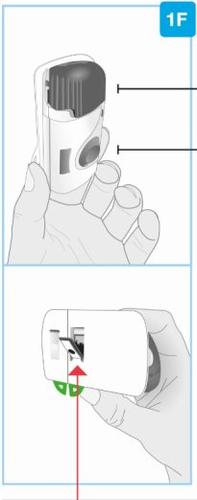
Inyector corporal de Skyrizi
Vista frontal Material adhesivo | Luz de estado | Botón de inicio No lo toque hasta que esté listo para inyectar |
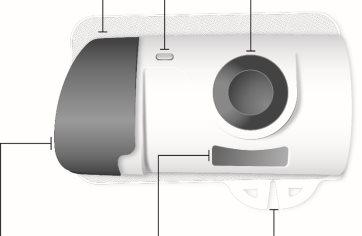
Puerta gris No cierre la puerta gris sin el cartucho en el interior | Ventana del medicamento | Pestañas |
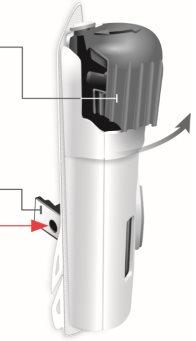
Vista posterior
Tira de plástico transparente | Protector de la aguja | Revestimiento adhesivo |
| Tenga cuidado. Aguja en el interior (debajo del protector de la aguja) No toque la zona del protector de la aguja ni la aguja | |
Pestaña verde de la sección pequeña | Pestaña verde de la sección grande |
Vista lateral
Cierre de la puerta El lado de la apertura tiene ranuras La puerta gris debe estar ligeramente abierta No cierre la puerta gris sin el cartucho en el interior Protector de la aguja Aguja en el interior (debajo del protector de la aguja) No toque la zona del protector de la aguja ni la aguja |
|
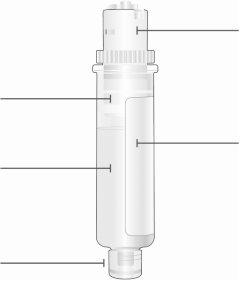
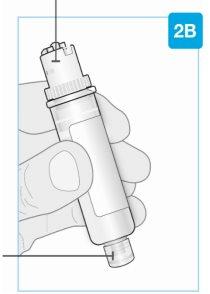
Cartucho
El émbolo blanco se desplaza por la cámara hacia la parte inferior del cartucho a medida que se inyecta el medicamento. Medicamento Punta inferior más pequeña |
| Parte superior más grande del cartucho No lo gire ni retire Fecha de caducidad (EXP) Se encuentra en la etiqueta del cartucho |
Información importante que debe conocer antes de la inyección de Skyrizi
- Usted debe haber recibido formación sobre cómo se inyecta Skyrizi antes de administrarse una inyección. Si necesita ayuda, consulte a su médico, farmacéutico o enfermero
- Marque las fechas en un calendario para saber cuándo le toca inyectarse Skyrizi
- El inyector corporal de un solo uso está diseñado para su uso únicamente con el cartucho de Skyrizi
- Conserve Skyrizi en su embalaje original para proteger el medicamento de la luz hasta el momento de utilizarlo
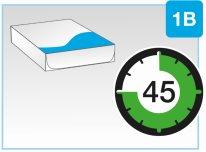
- Saque la caja de la nevera y déjela a temperatura ambiente, alejada de la luz solar directa, durante al menos 45 y hasta 90minutosantes de la inyección
- Nodeje que el inyector corporal se moje con agua ni con ningún otro líquido
- Notoque el botón de inicio hasta que coloque el inyector corporal cargado con el cartucho sobre la piel y esté listo para inyectar
- Únicamente puede pulsar el botón de inicio unasola vez
- Durante el proceso de inyección se debe limitar la actividad física. Se pueden realizar actividades físicas moderadas, como andar, estirarse o doblarse
- Noretrase la inyección del medicamento una vez cargado el cartucho limpio en el inyector corporal. Si se espera, el medicamento se secará y el inyector corporal dejará de funcionar
- Nose inyecte el medicamento si el líquido de la ventana de inspección está turbio o contiene escamas o partículas grandes. El líquido debe ser de transparente a color amarillo y puede contener partículas diminutas transparentes o blancas
- Noagite la caja, el cartucho ni el inyector corporal
- Noreutilice el cartucho ni el inyector corporal
Devuelva este medicamento a la farmacia
- después de la fecha de caducidad (EXP) indicada
- si el líquido se ha congelado en algún momento (incluso si se ha descongelado)
- si el cartucho o el inyector corporal se han caído o dañado
- si las perforaciones de la caja están rotas
- si la cubierta de papel blanco de la bandeja no está o se ha roto
Siga los siguientes pasos cada vez que utilice Skyrizi
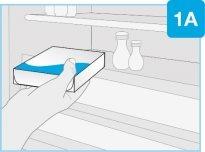
PASO1: Prepárese | |
| Saque la caja de la nevera y déjela a temperatura ambiente, alejada de la luz solar directa, durante al menos 45 y hasta 90minutosantes de la inyección.
|
Contenedor para residuos especiales
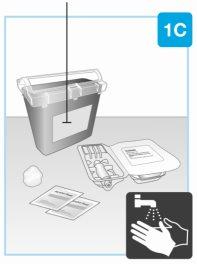
| Reúna todos los materiales y lávese las manos Sobre una superficie lisa y limpia, coloque lo siguiente:
Lávese y séquese las manos. |
| Retire el precinto de papel blanco de la bandeja
Levante la cubierta de plástico
|
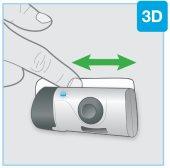
Puerta gris Botón de inicio Aguja en el interior(debajo del protector de la aguja) | Inspeccione el inyector corporal
Si se pulsa el botón de inicio gris antes de colocar el inyector en el cuerpo, ya no se podrá utilizar el inyector corporal. Si esto ocurre, informe a su médico, farmacéutico o enfermero. |
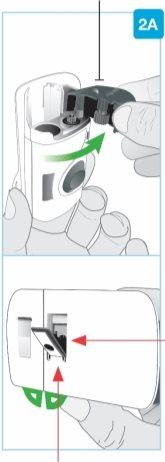
PASO2: Preparación del inyector corporal | |
Puerta gris
Vista posterior Aguja en el interior(debajo del protector de la aguja) Protector de la aguja | Abra totalmente la puerta gris
Deje el inyector corporal a un lado. |
Parte superior más grande del cartucho
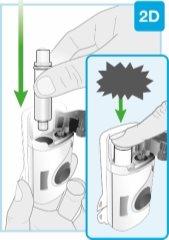
Punta inferior más pequeña | Inspeccione el cartucho Retire con cuidado el cartucho de la bandeja de plástico.
Compruebe el cartucho
|
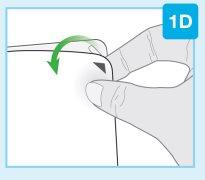
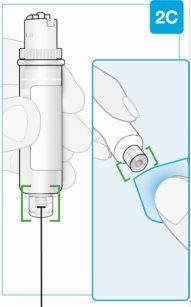
Punta inferior más pequeña Limpie el centro de la punta inferior más pequeña | Limpie la punta inferior más pequeña del cartucho Localice la punta inferior más pequeña del cartucho
|
Introduzca en línea recta “clic”
| Cargue el cartucho limpio en el inyector corporal
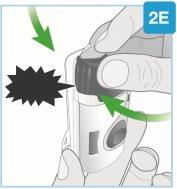
Asegúrese de proceder con el siguiente paso inmediatamente. Si se espera, se secará el medicamento. |
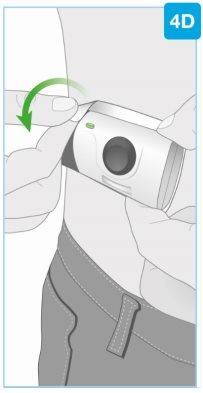
“chasquido” | Cierre la puerta gris Gire la puerta gris hacia la izquierda, luego apriete firmemente y escuchará un “chasquido” al cerrarse la puerta gris
|
PASO3: Preparación de la inyección | |
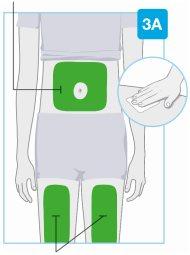
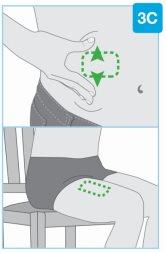
Zonas de inyección
Zonas de inyección | Escoja y limpie el lugar de inyección Escoja una de estas 3 zonas para ponerse la inyección:
Noinyecte en zonas de la piel con pliegues o prominencias naturales, ya que el inyector corporal podría caerse durante el uso. Antes de la inyección, limpie el lugar de inyección con una toallita impregnada en alcohol haciendo movimientos circulares.
|
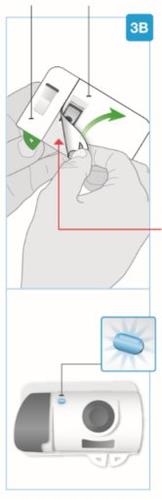
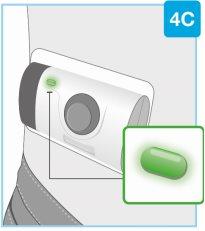
Sección pequeña Sección grande
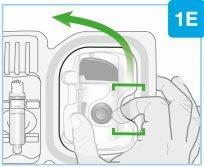
Aguja en el interior (debajo del protector de la aguja) Inyector activado La luz de estado parpadea en azul | Despegue ambas pestañas para que quede expuesto el adhesivo cutáneo Dé la vuelta al inyector corporal para localizar las dos pestañas verdes
Retire la sección grande utilizando la pestaña verde para que quede expuesto el adhesivo cutáneo Retire la sección pequeña utilizando la pestaña verde para que quede expuesto el adhesivo cutáneo. De este modo, se retirará la tira de plástico transparente y se activará el inyector corporal.
El inyector corporal de Skyrizi se debe colocar sobre la piel y la inyección se debe iniciar en los 30 minutos siguientes a la retirada de las pestañas verdes; de lo contrario, no funcionará. Asegúrese de proceder con el siguiente paso inmediatamente. |
| Si la luz de estado parpadea en rojo, el inyector corporal no funciona correctamente. No siga utilizándolo. Consulte a su médico, farmacéutico o enfermero para que le ayuden. Si el inyector corporal está pegado a su cuerpo, retírelo con cuidado de la piel. |
| Prepare el inyector corporal para su colocación
Asegúrese de colocar el inyector corporal de manera que pueda ver la luz de estado azul. Coloque el inyector corporal sobre la piel
Proceda inmediatamente con el siguiente paso. |
PASO4: Inyección de Skyrizi | |
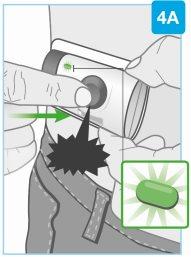
“clic” | Inicie la inyección Presione firmemente el botón gris de inicio y suéltelo
Nocontinúe utilizando el inyector corporal si la luz de estado parpadea en rojo. Retírelo con cuidado de la piel si la luz de estado parpadea en rojo. Si esto ocurre, informe a su médico, farmacéutico o enfermero. |
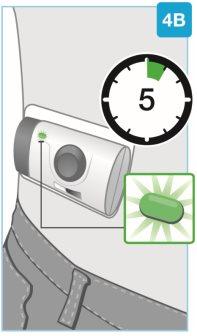
| Espere a que termine la inyección
Nosiga utilizando el inyector corporal si la luz de estado parpadea en rojo. Retírelo con cuidado de la piel si la luz de estado parpadea en rojo. Si esto ocurre, informe a su médico, farmacéutico o enfermero. |
| La inyección ha finalizado cuando:
|
| Retire el inyector corporal
Proceda con el siguiente paso. |
PASO5: Finalización | |
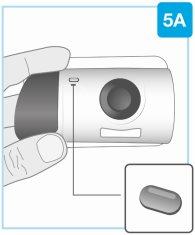
| Compruebe el inyector corporal Inspeccione la ventana del medicamento y la luz de estado. Compruebe que el émbolo blanco llena toda la ventana del medicamento y que la luz verde fija se apaga, lo que le indica que se ha inyectado todo el medicamento.
|
Contenedor para residuos especiales
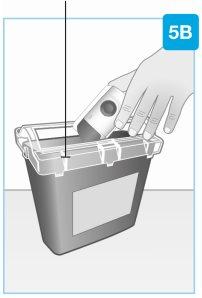
| Eliminación Tire el inyector corporal usado en un contenedor para residuos especiales inmediatamente después de su uso.
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SKYRIZI 180 MG SOLUCION INYECTABLE EN CARTUCHOForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: RisankizumabFabricante: Abbvie Deutschland Gmbh & Co. KgRequiere recetaForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: RisankizumabFabricante: Abbvie Deutschland Gmbh & Co. KgRequiere recetaForma farmacéutica: INYECTABLE, 360 MGPrincipio activo: RisankizumabFabricante: Abbvie Deutschland Gmbh & Co. KgRequiere receta
Médicos online para SKYRIZI 180 MG SOLUCION INYECTABLE EN CARTUCHO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SKYRIZI 180 MG SOLUCION INYECTABLE EN CARTUCHO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes