SIGNIFOR 0,3 MG SOLUCION INYECTABLE

Cómo usar SIGNIFOR 0,3 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Signifor0,3mg solución inyectable
Signifor 0,6mgsolución inyectable
Signifor 0,9mgsolución inyectable
pasireotida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, enfermero o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, enfermero o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Signifor y para qué se utiliza
- Qué necesita saber antes de empezar a usar Signifor
- Cómo usar Signifor
- Posibles efectos adversos
- Conservación de Signifor
- Contenido del envase e información adicional
1. Qué es Signifor y para qué se utiliza
Signifor es un medicamento que contiene el principio activo pasireotida. Se utiliza para tratar la enfermedad de Cushing en pacientes adultos para los que la cirugía no es una opción o para los que ha fallado la cirugía.
La enfermedad de Cushing está causada por un aumento del tamaño de la glándula pituitaria (una glándula situada en la base del cerebro) denominado adenoma de la pituitaria. Esto provoca que el cuerpo produzca una mayor cantidad de una hormona denominada hormona adenocorticotropa (ACTH), lo que a su vez provoca el aumento de la producción de otra hormona denominada cortisol.
El cuerpo humano produce de forma natural una sustancia denominada somatostatina, que bloquea la producción de ciertas hormonas, incluyendo la ACTH. La pasireotida funciona de forma muy similar a la somatostatina. Signifor es por lo tanto capaz de bloquear la producción de ACTH, ayudando a controlar la sobreproducción de cortisol y a mejorar los síntomas de la enfermedad de Cushing.
Si tiene alguna duda sobre cómo actúa Signifor o por qué le han prescrito este medicamento, consulte con su médico.
2. Qué necesita saber antes de empezar a usar Signifor
No use Signifor:
- si es alérgico a pasireotida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene problemas graves en el hígado.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Signifor si sufre o ha sufrido alguna vez:
- problemas con los niveles de azúcar en la sangre, o bien demasiado altos (como en la hiperglucemia/diabetes) o demasiado bajos (hipoglucemia);
- problemas del corazón como un ataque al corazón reciente, insuficiencia cardiaca congestiva (un tipo de enfermedad cardiaca donde el corazón no puede bombear suficiente cantidad de sangre por el cuerpo) o dolor repentino y opresivo en el pecho (normalmente se nota como presión, pesadez, opresión, compresión o dolor en todo el pecho);
- una alteración del ritmo del corazón, como un latido cardiaco irregular o una señal eléctrica anormal denominada «prolongación del intervalo QT», o «prolongación QT»;
- niveles bajos de potasio o magnesio en la sangre;
- cálculos biliares.
Durante su tratamiento con Signifor
- Signifor controla la sobreproducción de cortisol. El control puede ser demasiado fuerte y puede presentar signos o síntomas asociados con una falta de cortisol, como una debilidad extrema, cansancio, pérdida de peso, náuseas, vómitos o baja tensión arterial. Si le sucede esto, informe inmediatamente a su médico.
- Signifor puede causar un aumento del azúcar en la sangre. Su médico puede controlar su nivel de azúcar en la sangre y empezar el tratamiento o ajustar su medicamento antidiabético.
- Signifor puede reducir su ritmo cardiaco. Su médico puede controlar su ritmo cardiaco utilizando una máquina que mide la actividad eléctrica del corazón (un «ECG», o electrocardiograma). Si está utilizando un medicamento para tratar un problema del corazón, su médico también puede necesitar ajustar su dosis.
- su médico puede también vigilar periódicamente su vesícula biliar, los enzimas del hígado y las hormonas de la hipófisis, puesto que todos ellos se pueden ver afectados por este medicamento.
Niños y adolescentes
No dé este medicamento a niños y adolescentes menores de 18 años porque no hay datos disponibles en este grupo de edad.
Otros medicamentos y Signifor
Signifor puede afectar la manera de actuar de otros medicamentos. Si está utilizando otros medicamentos al mismo tiempo que Signifor (incluso medicamentos sin receta médica), su médico puede precisar controlar su corazón de forma más cuidadosa o bien cambiar la dosis de Signifor o de los otros medicamentos. Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. Especialmente, informe a su médico si está utilizando:
- medicamentos para tratar el ritmo cardiaco irregular, como aquellos que contienen disopiramida, procainamida, quinidina, sotalol, dofetilida, ibutilida, amiodarona o dronedarona;
- medicamentos para tratar las infecciones bacterianas (por vía oral: claritromicina, moxifloxacina; por vía inyectable: eritromicina, pentamidina);
- medicamentos para tratar infecciones por hongos (ketoconazol, excepto en champú);
- medicamentos para tratar algunas alteraciones psiquiátricas (clorpromacina, tioridazina, flufenazina, pimozida, haloperidol, tiaprida, amisulprida, sertindol, metadona);
- medicamentos para tratar la fiebre del heno y otras alergias (terfenadina, astemizol, mizolastina);
- medicamentos utilizados para la prevención o el tratamiento de la malaria (cloroquina, halofantrina, lumefantrina);
- medicamentos para controlar la presión arterial como:
- beta bloqueantes (metoprolol, carteolol, propranolol, sotalol)
- bloqueadores de los canales de calcio (bepridil, verapamilo, diltiazem)
- inhibidores de la colinesterasa (rivastigmina, fisostigmina);
- medicamentos para controlar el equilibrio de los electrolitos (potasio, magnesio) en el cuerpo.
Es particularmente importante que informe sobre alguno de estos medicamentos:
- ciclosporina (utilizado en el trasplante de órganos para reducir la actividad del sistema inmunitario);
- medicamentos para tratar los niveles de azúcar en sangre demasiado elevados (como en la diabetes) o demasiado bajos (hipoglucemia), tales como:
- insulina;
- metformina, liraglutida, vildagliptina, nateglinida (medicamentos antidiabéticos).
Embarazo, lactancia y fertilidad
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
- No debe utilizar Signifor durante el embarazo a menos que sea claramente necesario. Si está embarazada o piensa que puede estarlo, es importante informar a su médico, que decidirá con usted si puede utilizar Signifor durante su embarazo.
- No deberá dar el pecho mientras esté tomando Signifor. Se desconoce si Signifor pasa a la leche materna.
- Si es una mujer sexualmente activa, debe utilizar un método anticonceptivo efectivo durante el tratamiento. Consulte con su médico sobre la necesidad de anticoncepción antes de usar este medicamento.
Conducción y uso de máquinas
Signifor puede tener un pequeño efecto sobre la capacidad para conducir y utilizar máquinas, porque alguno de los efectos adversos que puede experimentar mientras utiliza Signifor, como mareo, dolor de cabeza y cansancio, pueden reducir su capacidad de conducir y utilizar máquinas de forma segura.
Información importante sobre alguno de los componentes de Signifor
Signifor contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Signifor
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Este medicamento se presenta en una ampolla, es decir un envase de vidrio pequeño.
Cuánto Signifor debe usar
La dosis recomendada es una ampolla de Signifor 0,6 mg dos veces al día. Utilizar Signifor a la misma hora cada día le ayudará a recordar cuándo debe usar su medicamento. Después de iniciar el tratamiento, su médico puede decidir aumentar la dosis a una ampolla de Signifor 0,9 mg dos veces al día.
Si aparecen efectos adversos su médico puede reducir la dosis de forma temporal en 0,3 mg por inyección.
Si tiene una enfermedad del hígado antes de iniciar el tratamiento con Signifor, su médico puede decidir iniciar el tratamiento con una dosis de una ampolla de Signifor de 0,3 mg dos veces al día.
Están disponibles ampollas de Signifor de diferentes dosis (0,3 mg, 0,6 mg y 0,9 mg) para administrar la dosis específica prescrita por su médico.
Su médico controlará regularmente cómo responde al tratamiento con Signifor y decidirá cuál es la dosis mejor para usted.
Cómo utilizar Signifor
Su médico o enfermero le enseñarán cómo debe inyectarse usted mismo Signifor. También deberá leer las instrucciones que figuran al final de este prospecto. Si tiene alguna duda, contacte con su médico, enfermero o farmacéutico.
Signifor se debe administrar por vía subcutánea. Esto significa que se inyecta con una aguja corta en el tejido graso que se encuentra justo debajo de la piel. Los muslos y el abdomen son unas zonas adecuadas para la inyección subcutánea. Utilizando un sitio diferente del de la inyección anterior para cada inyección se evitará el dolor y la irritación de la piel. También deberá evitar inyecciones en lugares que están doloridos o donde la piel está irritada.
No utilice Signifor si nota que la solución no es transparente o contiene partículas. La solución debe estar libre de partículas, transparente e incolora.
Durante cuánto tiempo utilizar Signifor
Debe continuar utilizando Signifor durante el tiempo que le indique su médico.
Si usa más Signifor del que debe
Si ha utilizado accidentalmente más Signifor del que le prescribió su médico, contacte inmediatamente con su médico, enfermero o farmacéutico.
Si olvidó usar Signifor
No se inyecte una dosis doble de Signifor para compensar las dosis olvidadas. Si olvidó inyectarse una dosis de Signifor, simplemente inyecte la próxima dosis a la hora que le toca.
Si interrumpe el tratamiento con Signifor
Si interrumpe su tratamiento con Signifor su nivel de cortisol puede aumentar otra vez y sus síntomas pueden aparecer de nuevo. Por lo tanto, no interrumpa el uso de Signifor a menos que se lo indique su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, enfermero o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves. Informe a su médico inmediatamente si experimenta alguno de los siguientes efectos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes)
- Cambio en el nivel de azúcar en la sangre. Puede notar una excesiva sed, eliminación de orina, aumento del apetito con pérdida de peso, cansancio, náuseas, vómitos, dolor abdominal.
- Cálculos biliares o complicaciones asociadas. Puede sufrir fiebre, temblores, color amarillento de la piel/ojos, dolor en la espalda repentino o dolor en el costado derecho del abdomen.
- Cansancio extremo.
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- Niveles bajos de cortisol. Puede presentar debilidad extrema, cansancio, pérdida de peso, náuseas, vómitos y baja presión arterial.
- Bajo ritmo cardiaco.
- Baja presión arterial. Puede experimentar mareos, ligera pesadez de cabeza y mareos o desvanecimiento al ponerse en pie.
- Problemas con el flujo de la bilis (colestasis). Puede presentar color amarillento en la piel, orina de color oscuro, heces claras y picor.
- Inflamación de la vesícula biliar (colecistitis).
Otros efectos adversos de Signifor pueden incluir:
Muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes)
- Diarrea
- Náuseas
- Dolor de estómago
- Dolor en el lugar de inyección
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- Intervalo QT prolongado (una señal eléctrica anormal en su corazón que se puede observar en las pruebas)
- Pérdida de apetito
- Vómitos
- Dolor de cabeza
- Mareo
- Caída del cabello
- Picor (prurito)
- Dolor muscular (mialgia)
- Dolor articular (artralgia)
- Resultados anómalos de los análisis de la función del hígado
- Resultados anómalos de los análisis de la función del páncreas
- Propiedades de la coagulación de la sangre anormales
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- Nivel bajo de glóbulos rojos (anemia)
Frecuencia no conocida (no se puede estimar a partir de los datos disponibles)
- Niveles elevados de cuerpos cetónicos (un grupo de sustancias producidas en el hígado) en la orina o la sangre (cetoacidosis diabética) como una complicación de un nivel elevado de azúcar en la sangre. Puede presentar aliento con olor afrutado, problemas para respirar y confusión.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Signifor
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la ampolla y en la caja después de «EXP»/«CAD». La fecha de caducidad es el último día del mes que se indica.
- Conservar en el embalaje original para protegerlo de la luz.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Signifor
- El principio activo es pasireotida.
Signifor 0,3 mg: Una ampolla de 1 ml de solución contiene 0,3 mg de pasireotida (como pasierotida diaspartato).
Signifor 0,6 mg: Una ampolla de 1 ml de solución contiene 0,6 mg de pasireotida (como pasireotida diaspartato).
Signifor 0,9 mg: Una ampolla de 1 ml de solución contiene 0,9 mg de pasireotida (como pasireotida diaspartato).
- Los demás componentes son manitol, ácido tartárico, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto de Signifor y contenido del envase
Signifor solución inyectable es una solución transparente e incolora en una ampolla. Cada ampolla contiene 1 ml de solución inyectable.
Signifor está disponible en envases que contienen 6 ampollas o en envases múltiples que contienen 18 (3 envases de 6), 30 (5 envases de 6) o 60 (10 envases de 6) ampollas.
Puede que, en su país, solamente estén comercializados algunas dosis y algunos tamaños de envases.
Titular de la autorización de comercialización
Recordati Rare Diseases
Immeuble Le Wilson
70 avenue du Général de Gaulle
92800 Puteaux
France
Responsable de la fabricación
Novartis Pharma GmbH
Roonstrasse 25
D‑90429 Nürnberg
Alemania
Recordati Rare Diseases
Immeuble Le Wilson
70 avenue du Général de Gaulle
92800 Puteaux
France
Recordati Rare Diseases
Eco River Parc
30 rue des Peupliers
92000 Nanterre
France
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Recordati Tél/Tel: +32 2 46101 36 | Lietuva Recordati AB. Tel: + 46 8 545 80 230 Švedija |
???????? Recordati Rare Diseases Te?.: +33 (0)1 47 73 64 58 ??????? | Luxembourg/Luxemburg Recordati Tél/Tel: +32 2 46101 36 Belgique/Belgien |
Ceská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francie | Magyarország Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franciaország |
Danmark Recordati AB. Tlf: + 46 8 545 80 230 Sverige | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 Franza |
Deutschland Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Nederland Recordati Tel: +32 2 46101 36 België |
Eesti Recordati AB. Tel: + 46 8 545 80 230 Rootsi | Norge Recordati AB. Tlf: + 46 8 545 80 230 Sverige |
Ελλ?δα Recordati Hellas Τηλ: +30 210 6773822 | Österreich Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Deutschland |
España Recordati Rare Diseases Spain S.L.U. Tel: + 34 91 659 28 90 | Polska Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francja |
France Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 | Portugal Jaba Recordati S.A. Tel: +351 21 432 95 00 |
Hrvatska Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 Francuska | România Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franta |
Ireland Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 France | Slovenija Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francija |
Ísland Recordati AB. Simi: + 46 8 545 80 230 Svíþjóð | Slovenská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francúzsko |
Italia Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Suomi/Finland Recordati AB. Puh/Tel : +46 8 545 80 230 Sverige |
Κ?προς Recordati Rare Diseases Τηλ : +33 1 47 73 64 58 Γαλλ?α | Sverige Recordati AB. Tel : +46 8 545 80 230 |
Latvija Recordati AB. Tel: + 46 8 545 80 230 Zviedrija | United Kingdom Recordati Rare Diseases UK Ltd. Tel: +44 (0)1491 414333 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
INSTRUCCIONESDEUSODESIGNIFOR SOLUCIÓN INYECTABLE
Este medicamento se presenta en una ampolla, es decir un envase de vidrio pequeño. Signifor se debe administrar utilizando jeringas y agujas para inyección desechables.
Su médico o enfermero le habrán dado instrucciones sobre cómo utilizar Signifor ampollas. Sin embargo antes de usar la ampolla, lea la siguiente información detalladamente. Si no está seguro sobre cómo administrarse usted mismo la inyección o si tiene cualquier duda, contacte con su médico o enfermero para obtener ayuda.
La inyección se puede preparar utilizando o bien dos agujas diferentes para retirar y para inyectar la solución o bien una aguja para inyección corta fina para estos dos pasos. En base a la práctica clínica local, su médico o enfermera le informarán sobre qué método utilizar. Por favor, siga sus instrucciones.
Conservar las ampollas de Signifor según las condiciones de almacenamiento que aparecen en la caja.
Información importante de seguridad
Precaución: Mantener las ampollas fuera del alcance de los niños.
Qué necesito
Para inyectarse usted mismo necesitará:
- Una ampolla de Signifor
- Una toallita con alcohol o similar
- Una jeringa estéril
- Una aguja estéril larga gruesa de punta roma para retirar la solución (su médico o enfermera le informarán si la necesita)
- Una aguja estéril corta fina
- Un contenedor de agujas u otro contenedor rígido cerrado para eliminar
Lugar de inyección
El lugar de inyección es el lugar de su cuerpo donde se va a administrar la inyección. Signifor se administra por vía subcutánea. Esto significa que se inyecta mediante una aguja corta en el tejido graso justo debajo de la piel. Los muslos y el abdomen son unas zonas adecuadas para la inyección subcutánea. Utilizando un sitio diferente del de la inyección anterior evitará el dolor y la irritación. También deberá evitar inyecciones en lugares que están doloridos o donde la piel esta irritada.
Preparación
Cuando esté preparado para administrarse usted mismo la inyección, siga cuidadosamente los siguientes pasos:
- Lávese las manos detenidamente con agua y jabón.
- Utilice jeringas y agujas desechables cada vez que se administre una inyección. Utilice jeringas y agujas sólo una vez. Nuncadebe compartir agujas ni jeringas.
- Saque la ampolla fuera de la caja.
- Inspeccione la ampolla. NO LA UTILICE si está rota o si el líquido se ve turbio o contiene partículas. En todos estos casos, devuelva el envase completo a la farmacia.
Para reducir la molestia local, se recomienda que la solución se mantenga a temperatura ambiente antes de la administración.
Las ampollas se deben abrir justo antes de la administración, y cualquier parte no utilizada se debe desechar.
Comprobar la fecha de caducidad y la dosis
Comprobar la fecha de caducidad que aparece en la etiqueta de la ampolla (después de «EXP») y comprobar que la ampolla contiene la dosis que su médico le ha prescrito.
NOLO UTILICE si el medicamento ha caducado o si la dosis no es correcta. En ambos casos, devuelva el envase completo a la farmacia.
Cómo inyectar Signifor
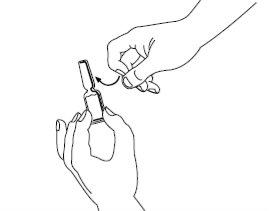
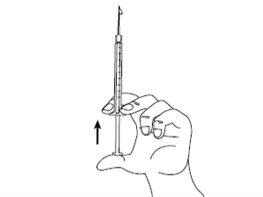
| Paso1: Signifor solución inyectable está contenido en una ampolla rompible. El punto de color en la parte superior indica la posición del punto de rotura en el cuello de la ampolla. Golpee ligeramente con el dedo la ampolla para asegurar que no haya líquido en la parte superior cuando la abra. |
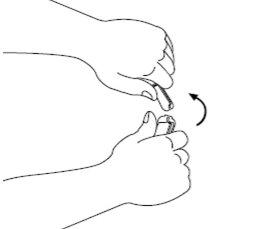
| Paso2: Procedimiento recomendado: mantenga la ampolla en posición vertical con el punto de color orientado hacia la cara opuesta. Sujete la base de la ampolla en una mano. Manteniendo juntos los pulgares por encima y debajo del cuello, rompa la parte superior de la ampolla por el punto de rotura. Una vez la ampolla esté abierta, colóquela en posición vertical sobre una superficie limpia y plana. |
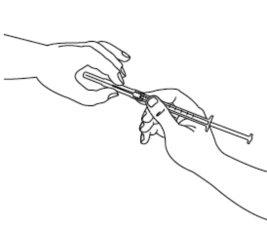
| Paso3: Coja la jeringa estéril e inserte la aguja. Si le han dado instrucciones para usar dos agujas, debe utilizar la aguja larga gruesa y de punta roma para este paso. Antes de proceder con el paso 4, limpie el lugar de inyección con una toallita de algodón. |
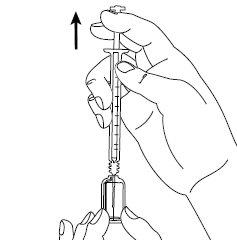
| Paso4: Quite la cubierta de la aguja. Introduzca la aguja en la ampolla y tire del émbolo para retirar el contenido completo de la ampolla a la jeringa. Si le han dado instrucciones para que utilice dos agujas, debe sustituir ahora la aguja larga por la aguja corta. |
| Paso5: Sujete la jeringa en una mano entre dos dedos colocando el dedo pulgar en la parte inferior del émbolo. Golpee ligeramente la jeringa con sus dedos para eliminar las burbujas de aire. Asegúrese que no hay burbujas de aire en la jeringa presionando el émbolo hasta que aparezca la primera gota en la punta de la aguja. No deje que la aguja toque nada. Ahora ya está preparado para la inyección. |
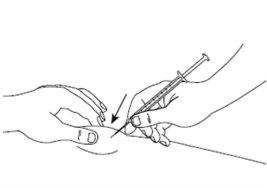
| Paso6: Pellizque suavemente la piel en el lugar de inyección y, manteniendo la aguja a un ángulo de aproximadamente 45 grados (tal como se muestra en la figura), introdúzcala en el lugar de inyección. Tire ligeramente del émbolo para comprobar que no se ha pinchado ningún vaso sanguíneo. Si observa sangre en la jeringa, primero retire la aguja de la piel, y después sustituya la aguja corta por otra nueva e insértela en un lugar de inyección diferente. |
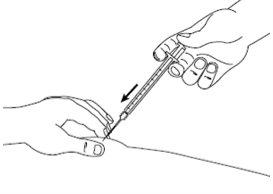
| Paso7: Siempre con la piel pellizcada, presione lentamente el émbolo hasta el final inyectando toda la solución. Mantenga el émbolo apretado y la jeringa en el sitio durante 5 segundos. |
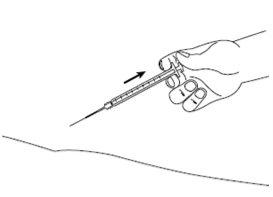
| Paso8: Suelte lentamente el pliegue de la piel y retire suavemente la aguja. Ponga de nuevo la cubierta en la aguja. |
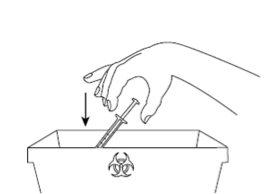
| Paso9: Deseche inmediatamente la jeringa y la aguja utilizada en un contenedor de agujas o en otro contenedor de eliminación rígido cerrado. La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SIGNIFOR 0,3 MG SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 0,3 mgPrincipio activo: PasireotidaFabricante: Recordati Rare DiseasesRequiere recetaForma farmacéutica: INYECTABLE, 0,6 mgPrincipio activo: PasireotidaFabricante: Recordati Rare DiseasesRequiere recetaForma farmacéutica: INYECTABLE, 0,9 mgPrincipio activo: PasireotidaFabricante: Recordati Rare DiseasesRequiere receta
Médicos online para SIGNIFOR 0,3 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SIGNIFOR 0,3 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes