
SICCAFLUID 2,5 mg/g GEL OFTALMICO EN UNIDOSIS

Cómo usar SICCAFLUID 2,5 mg/g GEL OFTALMICO EN UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
SICCAFLUID 2,5 mg/g GEL OFTÁLMICO EN UNIDOSIS
Carbómero 974 P
Lea todo el prospecto detenidamente porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si los síntomas empeoran o persisten, debe consultar al médico
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Contenido del prospecto
- Qué es Siccafluid y para qué se utiliza
- Antes de usar Siccafluid
- Cómo usar Siccafluid
- Posibles efectos adversos
- Conservación de Siccafluid
- Información adicional
1. Qué es Siccafluid y para qué se utiliza
Siccafluid es un sustituto de las lágrimas y contiene un lubricante denominado Carbómero 974P.
Se trata de un gel oftálmico usado para el alivio de los síntomas de la sequedad de ojos (como la inflamación, quemazón, irritación o sequedad) causados cuando los ojos no producen suficientes lágrimas.
2. Antes de usar SICCAFLUID
No use Siccafluid
•Si es alérgico (hipersensible) al carbómero o a cualquiera de los demás componentes de Siccafluid listados en la sección 6, “Qué contiene Siccafluid”.
Tenga especial cuidado con Siccafluid
•Si su condición empeora o no mejoradespués de haber iniciado el tratamiento con Siccafluid, contacte con su médico.
•NO INYECTAR, NO INGERIR.
Niños y adolescentes de hasta 18 años de edad
La seguridad y eficacia de SICCAFLUID en niños y adolescentes a la posología recomendada en adultos ha sido establecida en base a la experiencia clínica, pero no se dispone de estudios clínicos al respecto.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
Si tiene que utilizar cualquier otro medicamento oftálmico durante el tratamiento con Siccafluid: primero utilizar el otro medicamento oftálmico y esperar 15 minutos antes de utilizar Siccafluid .
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Si está embarazada o dando el pecho, contacte con su médico para consultarle antes de iniciar el uso de Siccafluid.
Él o ella decidirán si puede usar Siccafluid.
Conducción y uso de máquinas
Su vista puede volverse borrosa durante un corto tiempo después del uso de Siccafluid.
No deberá conducir o utilizar maquinaria hasta que su visión vuelva a la normalidad.
3. Cómo usar Siccafluid
Si le ha sido recomendado Siccafluid para su uso, siga exactamente las instrucciones de administración indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
La dosishabituales de 1 gota administrada en el ojo u ojos afectadoshasta4 veces al día.
Niños y adolescentes de hasta 18 años de edad
La seguridad y eficacia de SICCAFLUID en niños y adolescentes a la posología recomendada para adultos ha sido establecida en base a la experiencia clínica, pero no se dispone de estudios clínicos al respecto.
Cómo usar:
Lavarse las manos antes de abrir el envase unidosis.
Comprobar que el gel está en la punta del envase. Para abrir el envase girar hasta romper la pestaña de la parte superior.
Inclinar la cabeza hacia atrás y mirar hacia arriba.
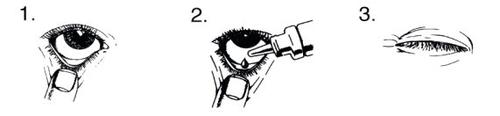
- Tirar ligeramente hacia abajo del párpado del ojo afectado hasta que se forme una pequeña “bolsa”
- Girar el envase boca abajo. Presionar hasta que caiga una gota en la “bolsa”.
- Suelte el párpado inferior y parpadee varias veces.
- Repita los pasos 1 a 3 en el otro ojo si también necesita tratarse.
Para ayudar a prevenir la infección no tocar el ojo, las zonas de alrededor o cualquier otra con la punta del envase.
Desechar el envase unidosis después de su utilización. No lo guarde para utilizarlo más tarde.
Si usa más Siccafluid del que debiera
El uso de más gotas de Siccafluid de las que debiera no causa ningún daño.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servivio de Información Toxicológica, teléfono: 91 562 04 20.
Si olvidó usar Siccafluid
No use una dosis doble para compensar la dosis olvidada. Aplicar la siguiente dosis según la frecuencia que tenga establecida.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Siccafluid puede producir efectos adversos, aunque no todas las personas los sufran.
Contactecon su médico si:
•Los síntomas empeoran o no mejoran habiendo iniciado el tratamiento con Siccafluid.
Si sufre alguno de los siguientes efectos adversos después de aplicar el gel oftálmico en el ojo, hable con su médico si ello le preocupa:
•Visión borrosa pasajera
•Leve picazón o sensación de quemazón en el ojo.
Los efectos adversos mencionados pueden ocurrir, pero el número de personas con probabilidad de ser afectadas puede variar.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Siccafluid
Mantener fuera del alcance y de la vista de los niños.
No utilice Siccafluid 2,5 mg/g gel oftálmico después de la fecha de caducidad que aparece en el envase y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC. Conservar los envases unidosis en el embalaje original para protegerlos de la luz.
Desechar inmediatamente el envase unidosis con la solución restante después de su uso.
No guardar para usarlo más tarde.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita.De esta forma,ayudará a proteger el medio ambiente.
6. Información adicional
Composición de Siccafluid
- El principio activo es Carbómero 974 P 2,5mg/g.
- Los demás componentes son sorbitol, monohidrato de lisina, acetato de sodio trihidratado, alcohol polivinílico y agua para inyectables.
Aspecto del producto y contenido del envase
Siccafluid es un gel opalescente ligeramente amarillento en envases unidosis.
Cada envase unidosis contiene 0,5 g de gel.
Cada caja contiene 10, 20,30 o 60 envases unidosis (no todas las presentaciones pueden estar comercializadas)
Titular de la autorización de comercialización
LABORATORIOS THEA S.A.
C/ Enric Granados, nº 86-88, 2ª planta, 08008 Barcelona
Responsable de la fabricación
LABORATOIRES UNITHER
1 RUE DE L’ARQUERIE 50200 COUTANCES
FRANCIA
O
LABORATOIRES UNITHERESPACE INDUSTRIEL NORD
151, RUE ANDRÉ DUROUCHEZ – BP 28028
80084 AMIENS CEDEX-2
FRANCIA
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Francia, España, Italia y Portugal:……………………………………..SICCAFLUID
Holanda………………………………………………………SICCAFLUID UNIDOSE
Islandia y Noruega……………………………………………………..OFTAGEL
Este prospecto ha sido aprobado en Marzo 2015
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.es/
- País de registro
- Precio medio en farmacia7.02 EUR
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SICCAFLUID 2,5 mg/g GEL OFTALMICO EN UNIDOSISForma farmacéutica: COLIRIO, 5,5 mg sodio cloruro;3 mg hipomelosa/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Alcon Healthcare S.A.No requiere recetaForma farmacéutica: COLIRIO, 3,2 mg/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Bausch & Lomb S.A.No requiere recetaForma farmacéutica: COLIRIO, 3,2 mg/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Bausch & Lomb S.A.No requiere receta
Médicos online para SICCAFLUID 2,5 mg/g GEL OFTALMICO EN UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SICCAFLUID 2,5 mg/g GEL OFTALMICO EN UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















