RINOBANEDIF POMADA NASAL


Cómo usar RINOBANEDIF POMADA NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Rinobanedif Pomada Nasal
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4. |
Contenido del prospecto
- Qué es Rinobanedif y para qué se utiliza
- Qué necesita saber antes de empezar a usar Rinobanedif
- Cómo usar Rinobanedif
- Posibles efectos adversos
- Conservación de Rinobanedif
- Contenido del envase e información adicional
1. Qué es Rinobanedif y para qué se utiliza
Es una pomada nasal que contiene como principios activos dos antibióticos (bacitracina y neomicina), un corticosteroide, prednisolona (antiinflamatorio), fenilefrina (descongestionante nasal), clorobutanol (antiséptico) y dos componentes con propiedades aromatizantes (aceite de eucalipto y esencia de niaulí); en aplicación en la mucosa nasal Rinobanedif proporciona actividad antiséptica, antiinflamatoria y vasoconstrictora.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas como la gripe o el catarro.
Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico.
No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura.
Rinobanedif está indicado en el tratamiento local de afecciones de la mucosa nasal que cursen con congestión, inflamación (como algunos síntomas de rinitis) y/o pequeñas heridas, con o sin formación de costras, en adultos y niños mayores de 6 años.
2. Qué necesita saber antes de empezar a usar Rinobanedif
No use Rinobanedif:
- Si es alérgico a los principios activos o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si padece afecciones tuberculosas, sífilis, afecciones por hongos (fúngicas) o por virus (por ej., herpes o varicela), incluido en las vías respiratorias.
- En los ojos ni en heridas abiertas.
- En niños menores de 6 años.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Rinobanedif.
- Administrar con precaución en las siguientes situaciones: enfermedad arterial coronaria o cardiaca, hipertensión, latidos irregulares (arritmias), enfermedad de tiroides, diabetes mellitus, dificultad al orinar por problemas de próstata, glaucoma (aumento de la presión dentro de los ojos), úlceras recientes en el tabique nasal, cirugía nasal reciente o traumatismo nasal reciente.
- Debido al contenido del corticoide (prednisolona) por vía nasal, se pueden producir cualquiera de los efectos adversos que se han notificado sobre el uso de corticosteroides por vía oral, especialmente cuando se utilizan a dosis altas y en tratamientos prolongados, como la alteración de unas glándulas localizadas junto a los riñones que produce la aparición de síntomas como obesidad, retraso en el crecimiento en niños, etc. (síndrome de Cushing).
- No debe usar el medicamento por tiempo prolongado.
- Si es hipersensible a ciertos antibióticos (aminoglucósidos) puede ser sensible a neomicina y si es sensible a ésta puede ser sensible a bacitracina, componentes de este medicamento.
Niños
No administrar a niños menores de 6 años, por no haberse establecido la seguridad y eficacia de este medicamento en ellos.
Los niños tienen más propensión a tener efectos adversos por corticosteroides que los adultos.
Otros medicamentos y Rinobanedif
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento, incluso los adquiridos sin receta.
Algunos medicamentos pueden aumentar los efectos de Rinobanedif, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Si se produce suficiente absorción interna puede dar lugar a crisis hipertensivas y arritmias cardíacas, especialmente cuando se administra junto a IMAO (antidepresivos y para otros usos), antidepresivos tricíclicos y beta-bloqueantes (para el corazón). También se puede producir interacción con maprotilina (antidepresivo) y otros aminoglucósidos (antibióticos).
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Dado que alguno de los componentes de Rinobanedif podría absorberse a la circulación, sobre todo con administración en gran cantidad y de forma prolongada, no debe administrarse durante el embarazo, salvo que el médico considere que el beneficio para la madre supera los posibles riesgos para el feto o el bebé. No aplicar Rinobanedif durante la lactancia. |
Conducción y uso de máquinas
No se han descrito efectos sobre la capacidad para conducir y utilizar máquinas.
3. Cómo usar Rinobanedif
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
La dosis recomendada es:
Adultos y niños mayores de 6 años:Realizar de 1 a 3 aplicaciones al día.
La duración media del tratamiento será de 5 días.
Uso en niños
No administrar a niños menores de 6 años.
Vía nasal.
Instrucciones para el uso correcto de la pomada
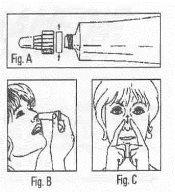
Apertura del tubo y modo de empleo: Para abrir el tubo, desenrosque el conjunto tapón-cánula y retire la arandela de seguridad (Fig. A). Coloque de nuevo la cánula, enroscándola hasta el fondo, para así perforar el precinto alumínico del tubo. Aplicar en ambos orificios nasales, una pequeña cantidad de Rinobanedif (Fig. B) procurando una distribución uniforme de la pomada, para lo que es conveniente un ligero masaje externo. (Fig. C). |
|
Después de la aplicación, limpiar el extremo del tubo con un paño limpio y húmedo. No utilizar el envase por más de una persona.
Debe consultar al médico si empeora o no mejora después de 7 días de tratamiento.
Si usa más Rinobanedifdel que debe
Dada la forma de administración, resulta muy difícil la intoxicación.
En caso de sobredosificación, los efectos que pueden producirse serían fundamentalmente irritación local, y en caso de absorción suficiente de fenilefrina, aparición de alteraciones cardiovasculares (arritmias cardíacas, hipertensión).
En el caso de ingestión accidental, un envase, existiría irritación gástrica y no tendría por qué haber en principio alteraciones cardiovasculares.
El tratamiento, en caso de sobredosificación o ingestión accidental, deberá incluir el control de la tensión arterial y electrocardiograma. Además, en caso de ingesta (superior o igual a un envase), se provocará el vómito o se realizará un lavado gástrico y se administrará un protector de mucosa.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico, o acuda a un centro médico, o llame al Servicio de Información Toxicológica, Tel. 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó usar Rinobanedif
No use una dosis doble para compensar la dosis olvidada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
La valoración de los efectos adversos se basa en las siguientes frecuencias:
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
Frecuentes: pueden afectar hasta 1 de cada 10 personas
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
Raras: pueden afectar hasta 1 de cada 1.000 personas
Muy raras: pueden afectar hasta 1 de cada 10.000 personas.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles).
Durante el uso de Rinobanedif pomada pueden aparecer los siguientes efectos adversos:
- Frecuentes: se podría producir dermatitis de contacto alérgica (inflamación en la zona de aplicación); después del uso continuado o excesivo puede aparecer una congestión nasal de rebote.
- Con frecuencia rara: se han observado reacciones alérgicas de tipo cutáneo: enrojecimiento, picor, sequedad o sensación de quemazón de la mucosa nasal. En raras ocasiones también: estornudos y signos de absorción interna tales como: dolor de cabeza, vértigo o nerviosismo, latidos irregulares, hipertensión y reacción anafiláctica (reacción alérgica exagerada), en general si se aplicase la pomada en lesiones profundas.
- En muy raras ocasiones: se puede producir ototoxicidad (pérdida de audición), nefrotoxicidad (afectación del riñón), especialmente si existe disfunción renal, ligera hemorragia nasal, sequedad nasal e irritación.
Los efectos adversos de los corticosteroides aumentan con factores que aumentan la absorción, como son el uso prolongado, en áreas extensas o con materiales oclusivos.
Con el uso de corticosteroides tópicos, en particular por vía nasal, se podrían producir además los siguientes efectos adversos:
Trastornos del olfato y del gusto; raramente, ulceración o perforación del tabique nasal.
Otros efectos adversos de los corticoides son: atrofia de la piel, cambios en el color de la piel, propensión a tener hematomas, aparición de vasos sanguíneos bajo la superficie de la piel, inflamación de folículos pilosos (foliculitis), aumento del vello, estrías, acné, infección secundaria como infección por hongos.
Efectos adversos debidos a absorción del corticoide: afección caracterizada por obesidad, cara redondeada, acumulación de grasa en la zona cervical, retraso en la cicatrización, síntomas psiquiátricos, etc. (síndrome de Cushing); cataratas, glaucoma.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rinobanedif
No requiere condiciones especiales de conservación.
Este medicamento debe usarse en 6 meses después de su apertura.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Rinobanedif
- Los principios activos son: bacitracina-zinc, neomicina sulfato, prednisolona, fenilefrina hidrocloruro, clorobutanol hemihidrato, aceite de eucalipto y esencia de niaulí. Cada gramo de pomada contiene: 500 UI de bacitracina zinc, 5 mg de neomicina sulfato (equivalente a 3,5 mg de neomicina base), 3 mg de prednisolona (0,3%), 2,5 mg de fenilefrina hidrocloruro (0,25%), 8 mg de clorobutanol hemihidrato, 2,00 mg - 2,86 mg de aceite de eucalipto (equivalente a 2 mg de 1,8 Cineol) y 1,54 mg - 2,22 mg de esencia de niaulí (equivalente a 1 mg de 1,8 Cineol).
- Los demás componentes (excipientes) son: colesterol, plastibase, vaselina blanca filante y vaselina líquida.
Aspecto del producto y contenido del envase
Rinobanedif es una pomada nasal; es untuosa, de color blanco amarillenta.
Cada envase contiene un tubo de aluminio con 10 g de pomada nasal.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
TEOFARMA S.R.L.
Via F.lli Cervi, 8
27010 Valle Salimbene (PV) - Italia
Responsable de la fabricación:
DOPPEL FARMACEUTICI, S.R.L.
Via Martiri delle Foibe, 1. Cortemaggiore
(Piacenza)- 29016- Italia
Fecha de la última revisión de este prospecto:Febrero 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RINOBANEDIF POMADA NASALForma farmacéutica: PRODUCTO USO NASAL, 27,5 µgPrincipio activo: fluticasona furoatoFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: PRODUCTO USO NASAL, 27,5 MICROGRAMOS/PULVERIZACIÓNPrincipio activo: fluticasona furoatoFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 137 microgramos/50 microgramos/aplicaciónPrincipio activo: fluticasone, combinationsFabricante: Laboratorios Cinfa S.A.Requiere receta
Médicos online para RINOBANEDIF POMADA NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RINOBANEDIF POMADA NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes