
REKOVELLE 36 MICROGRAMOS/1,08 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar REKOVELLE 36 MICROGRAMOS/1,08 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
REKOVELLE 36microgramos/1,08ml
solución inyectable en pluma precargada
folitropina delta
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es REKOVELLE y para qué se utiliza
- Qué necesita saber antes de usar REKOVELLE
- Cómo usar REKOVELLE
- Posibles efectos adversos
- Conservación de REKOVELLE
- Contenido del envase e información adicional
1. Qué es REKOVELLE y para qué se utiliza
REKOVELLE contiene folitropina delta, la hormona folículo estimulante que pertenece a la familia de las hormonas llamadas gonadotropinas. Las gonadotropinas están implicadas en la reproducción y la fertilidad.
REKOVELLE se usa en el tratamiento de la infertilidad femenina y en mujeres sometidas a técnicas de reproducción asistida tales como la fecundación in vitro(FIV) y la inyección intracitoplasmática de espermatozoides (ICSI). REKOVELLE estimula los ovarios para que crezcan y desarrollen numerosos sacos (‘folículos’), de los cuales se obtienen los óvulos que se fecundan en el laboratorio.
2. Qué necesita saber antes de usar REKOVELLE
Antes de comenzar el tratamiento con este medicamento, un médico debe evaluarle a usted y a su pareja para examinar las posibles causas del problema de infertilidad.
No use REKOVELLE
- si es alérgico a la hormona folículo estimulante o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene un tumor en el útero, ovarios, mamas, hipófisis o hipotálamo
- si presenta aumento de los ovarios o quistes no provocados por el síndrome de ovario poliquístico
- si presenta sangrado vaginal de causa desconocida
- si ha tenido menopausia precoz
- si tiene malformaciones en los órganos sexuales que hagan imposible un embarazo normal
- si tiene miomas en el útero que hagan imposible un embarazo normal.
Advertencias y precauciones
Consulte con su médico antes de usar REKOVELLE
Síndrome de hiperestimulación ovárica
Las gonadotropinas, como este medicamento, pueden producir síndrome de hiperestimulación ovárica. Esto se produce cuando sus folículos se desarrollan en exceso y se convierten en grandes quistes.
Consulte con su médico si:
- presenta dolor, molestia o hinchazón abdominal
- presenta náuseas
- presenta vómitos
- presenta diarrea
- presenta ganancia de peso
- tiene dificultad para respirar
Su médico puede pedirle que interrumpa el tratamiento con este medicamento (ver sección 4).
Si se sigue la dosis y la pauta de administración recomendadas, es menos probable que se produzca el síndrome de hiperestimulación ovárica.
Problemas en la coagulación (acontecimientos tromboembólicos)
La formación de coágulos dentro de los vasos sanguíneos (venas o arterias) es más probable en mujeres embarazadas. Los tratamientos de infertilidad pueden aumentar el riesgo de que esto ocurra, especialmente si usted tiene sobrepeso o usted o alguien de su familia (pariente de sangre) tiene un problema de coagulación conocido (trombofilia). Consulte con su médico si piensa que esto es aplicable a usted.
Retorcimiendo de ovarios (torsión ovárica)
Se han comunicado casos de retorcimiento de los ovarios (torsión ovárica) tras un tratamiento de reproducción asistida. El retorcimiento de los ovarios puede cortar el flujo sanguíneo que llega a los ovarios.
Embarazo múltiple y defectos de nacimiento
Cuando se está sometida a un tratamiento con técnicas de reproducción asistida, la posibilidadde tener un embarazo múltiple (como gemelos) se relaciona principalmente con el número de embriones que se depositen en su útero, la calidad de los embriones y su edad. El embarazo múltiple puede conducir a complicaciones médicas para usted y sus bebés. Además el riesgo de defectos de nacimiento puede ser ligeramente mayor cuando se siguen tratamientos de infertilidad, se piensa que esto es debido a las características de los padres (como su edad, y las características del esperma de su pareja) y el embarazo múltiple.
Pérdida del embarazo
Cuando se sigue un tratamiento con técnicas de reproducción asistida, es más probable que se produzca un aborto espontáneo que con la concepción natural.
Embarazo fuera del útero (embarazo ectópico)
Cuando se sigue un tratamiento con técnicas de reproducción asistida, es más probable que tenga un embarazo fuera del útero (embarazo ectópico) que con la concepción natural. Si tiene antecedentes de enfermedad tubárica, el riesgo de embarazo ectópico, está aumentado en su caso.
Tumores ováricos y otros tumores del sistema reproductor
Se han comunicado casos de tumores ováricos y de otros tumores del sistema reproductor en mujeres que han estado sometidas a tratamientos de infertilidad. No se conoce si el tratamiento con medicamentos para la fertilidad incrementan el riesgo de estos tumores en mujeres estériles.
Otras condiciones médicas
Antes de empezar a usar este medicamento, avise a su médico si:
- otro médico le ha dicho que el embarazo podría ser peligroso para usted.
- usted tiene enfermedad del hígado o del riñón
Niños y adolescentes (por debajo de los 18años de edad)
Este medicamento no está indicado para su uso en niños y adolescentes.
Otros medicamentos y REKOVELLE
Informe a su médico si está usando, ha usado recientemente, o podría tener que usar cualquier otro medicamento.
Embarazo y lactancia
No use este medicamento si está embarazada o en periodo de lactancia.
Conducción y uso de máquinas
Este medicamento no afecta a su capacidad para conducir y utilizar máquinas.
REKOVELLE contiene sodio
Este medicamento contiene menos de 1 mmol de cloruro sódico (23 mg) por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar REKOVELLE
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis de REKOVELLE para el primer ciclo de tratamiento será calculada por su médico usando el nivel de hormona anti-Muleriana (HAM– un marcador de como van a responder sus ovarios a la estimulación con gonadotropinas) – en su sangre y el peso corporal. Por tanto, antes del comienzo del tratamiento, se debe disponer del resultado de la AMH de una muestra de sangre (tomada en los últimos 12 meses). Se medirá también su peso corporal antes del comienzo del tratamiento. La dosis de REKOVELLE se expresa en microgramos.
La dosis de REKOVELLE se fija a lo largo del periodo de tratamiento, sin ajustes para aumentar o disminuir la dosis diaria. Su médico monitorizará el efecto del tratamiento con REKOVELLE, y el tratamiento se suspenderá cuando se produzca un número adecuado de folículos. En general, se le administrará una inyección única de un medicamento llamado gonadotropina coriónica humana (hCG) con una dosis de 250 microgramos o de 5 000 UI para el desarrollo final de los folículos.
Si la respuesta de su cuerpo al tratamiento es muy débil o muy fuerte, su médico puede decidir suspender el tratamiento con REKOVELLE. Para el siguiente ciclo de tratamiento, su médico podrá darle una dosis diaria de REKOVELLE más alta o más baja que la anterior.
Cómo se administran las inyecciones
Las instrucciones de uso de la pluma precargada deben seguirse cuidadosamente. No use la pluma precargada si la solución contiene partículas o no parece transparente.
La primera inyección de este medicamento debe administrarse bajo la supervisión de un médico o enfermero. Su médico decidirá si usted puede auto-administrarse este medicamento en casa, pero sólo tras haber recibido el entrenamiento adecuado.
Este medicamento está indicado para su administración por inyección justo debajo de la piel (subcutánea) normalmente en el abdomen. La pluma precargada puede utilizarse para varias inyecciones.
Si usa más REKOVELLE del que debe
Los efectos de usar demasiado medicamento no se conocen. Es posible que se produzca el síndrome de hiperestimulación ovárica (SHO), que está descrito en la sección 4.
Si olvidó usar REKOVELLE
No se administre una dosis doble para compensar las dosis olvidadas. Por favor, contacte con su médico o farmacético tan pronto como se de cuenta de que ha olvidado una dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves:
Las hormonas que se usan en el tratamiento de la infertilidad, como este medicamento, pueden causar altos niveles de actividad en los ovarios (Síndrome del Hiperestimulación Ovárica).Los síntomas pueden incluir dolor, molestia e hinchazón en el abdomen, náusea, vómito, diarrea, ganancia de peso o dificultad respiratoria. Si tiene cualquiera de estos síntomas, por favor contacte inmediatamente con su médico.
El riesgo de reacciones adversas se clasifica en las siguientes categorías:
Frecuentes (pueden afectar hasta 1 de cada 10personas):
- Dolor de cabeza
- Náuseas
- Síndrome de hiperestimulación ovárica (ver arriba)
- Dolor pélvico y molestia, incluido el que se origina en los ovarios
- Cansancio (fatiga)
Poco frecuentes (pueden afectar hasta 1 de cada 100personas):
- Cambios de humor
- Somnolencia / Adormecimiento
- Mareo
- Diarrea
- Vómitos
- Estreñimiento
- Molestia en el abdomen
- Hemorragia vaginal
- Molestias en las mamas (incluyen dolor mamario, hinchazón mamaria, sensibilidad mamaria y/o dolor en el pezón)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de REKOVELLE
Mantener fuera de la vista y del alcance de los niños.
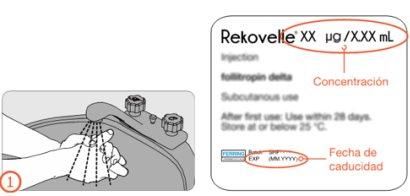
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la pluma precargada y el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (2 °C – 8 °C). No congelar.
Consérvese en el embalaje original para protegerlo de la luz.
REKOVELLE puede conservarse a o por debajo de 25 °C hasta 3 meses, incluyendo el periodo después del primer uso. No debe ser refrigerado de nuevo y debe ser desechado si no se ha usado después de 3 meses.
Después del primer uso: 28 días cuando se conserve a o por debajo de 25 °C.
Al finalizar el tratamiento se debe desechar el producto no utilizado.
Los medicamentos no se deben tirar por los desagües ni a la basura. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de REKOVELLE
- La sustancia activa es folitropina delta.
Cada pluma precargada con cartucho multidosis contiene 36 microgramos de folitropina delta en 1,08 mililitros de solución. Un mililitro de solución contiene 33,3 microgramos de folitropina delta en cada mililitro de solución.
- El resto de componentes son fenol, polisorbato 20, L-metionina, sulfato de sodio decahidrato, fosfato de disodio dodecahidrato, ácido fosfórico concentrado, hidróxido de sodio y agua para inyección.
Aspecto del producto y contenido del envase.
REKOVELLE es una solución para inyección transparente e incolora en pluma precargada. Está disponible en envases de 1 pluma precargada y 9 agujas.
Titular de la autorización de comercialización
Ferring Pharmaceuticals A/S
Amager Strandvej 405
2770 Kastrup
Denmark
Responsable de la fabricación
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Ferring N.V. Tel/Tél: +32 53 72 92 00 | Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
???????? ??????? ???? ???: +359 2 807 5022 | Luxembourg/Luxemburg Ferring N.V. Belgique/Belgien Tel/Tél: +32 53 72 92 00 |
Ceská republika Ferring Pharmaceuticals CZ s.r.o. Tel: +420 234 701 333 | Magyarország Ferring Magyarország Gyógyszerkereskedelmi Kft. Tel: +36 1 236 3800 |
Danmark Ferring Lægemidler A/S Tlf: +45 88 16 88 17 | Malta E.J. Busuttil Ltd. Tel: +356 21447184 |
Deutschland Ferring Arzneimittel GmbH Tel: +49 431 5852 0 | Nederland Ferring B.V. Tel: +31 235680300 |
Eesti CentralPharma Communications OÜ Tel: +372 601 5540 | Norge Ferring Legemidler AS Tlf: +47 22 02 08 80 |
Ελλ?δα Ferring Ελλ?ς ΜΕΠΕ Τηλ: +30 210 68 43 449 | Österreich Ferring Arzneimittel Ges.m.b.H Tel: +43 1 60 8080 |
España Ferring S.A.U. Tel: +34 91 387 70 00 | Polska Ferring Pharmaceuticals Poland Sp. z o.o. Tel: +48 22 246 06 80 |
France Ferring S.A.S. Tél: +33 1 49 08 67 60 | Portugal Ferring Portuguesa – Produtos Farmacêuticos, Sociedade Unipessoal, Lda. Tel: +351 21 940 51 90 |
Hrvatska Clinres farmacija d.o.o. Tel: +385 1 2396 900 | România Ferring Pharmaceuticals Romania SRL Tel: +40 356 113 270 |
Ireland Ferring Ireland Ltd. Tel: +353 1 4637355 | Slovenija SALUS, Veletrgovina, d.o.o. Tel: +386 1 5899 100 |
Ísland Vistor hf. Sími: +354 535 70 00 | Slovenská republika Ferring Slovakia s.r.o. Tel: +421 2 54 416 010 |
Italia Ferring S.p.A. Tel: +39 02 640 00 11 | Suomi/Finland Ferring Lääkkeet Oy Puh/Tel: +358 207 401 440 |
Κ?προς A.Potamitis Medicare Ltd Τηλ: +357 22583333 | Sverige Ferring Läkemedel AB Tel: +46 40 691 69 00 |
Latvija CentralPharma Communications SIA Talr: +371 674 50497 | United Kingdom(Northern Ireland) Ferring Ireland Ltd Tel: +353 1 4637355 |
Fecha de la última revisión de este prospecto en .
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Europea del Medicamento (EMA): http://www.ema.europa.eu.
Instrucciones de uso
Pluma precargada de REKOVELLE
folitropina delta
Su profesional sanitario debe enseñarle como preparar e inyectarse REKOVELLE de forma adecuada antes de inyectárselo por primera vez.
No intente inyectárselo usted misma hasta que no haya sido entrenada por su profesional sanitario en la forma correcta de administrarse las inyecciones.
Lea este manual completo antes de usar la pluma precargada de REKOVELLE y cada vez que adquiera una nueva pluma. Podría haber nueva información. Siga cuidadosamente estas instrucciones incluso si ha usado una pluma inyectable similar antes. El uso incorrecto de la pluma podría dar como resultado una dosis incorrecta del medicamento.
Contacte con su profesional sanitario (médico, enfermero o farmacéutico) si tiene cualquier duda sobre cómo administrarse su inyección de REKOVELLE.
La pluma precargada de REKOVELLE es una pluma desechable, dosificadora que puede utilizarse para la administración de más de 1 dosis de REKOVELLE. La pluma está disponible en 3 concentraciones diferentes:
- 12 microgramos/0.36 mL
- 36 microgramos/1.08 mL
- 72 microgramos/2.16 mL
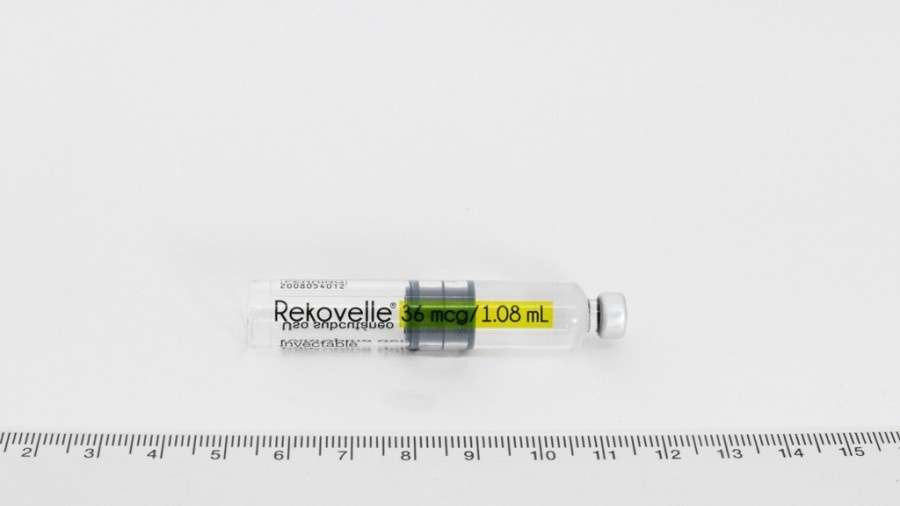
Pluma precargada de REKOVELLE y sus partes
Instrucciones de uso – pluma precargada de REKOVELLE (folitropina delta)
Información importante
- La pluma precargada de REKOVELLE y las agujas son para uso por una sóla persona y no deben compartirse con otros.
- Use la pluma solo para la condición médica que se le haya prescrito y cómo su profesional sanitario le haya instruído.
- Si es ciega o tiene una visión deficiente y no puede leer la escala de dosis en la pluma, no use esta pluma sin ayuda. Pida ayuda a una persona con buena visión y que esté entrenada en cómo utilizar la pluma.
- Si tiene alguna pregunta, contacte con su profesional sanitario o con el representante local del titular de la autorización de comercialización (consulte el prospecto para la información de contacto) antes de administrarse la inyección de REKOVELLE.
Información acerca de su pluma precargada de REKOVELLE
La pluma puede graduarse para la administración de dosis desde 0.33 microgramos a 20 microgramos de REKOVELLE en incrementos marcados de 0.33 microgramos. Ver “Ejemplos de como marcar una dosis” en las páginas 20 a 211.
- La escala de dosis de la pluma está numerada de 0 a 20 microgramos.
- Cada número está separado por dos líneas, cada línea equivale a un incremento de 0.33 microgramos.
- Cuando gire el marcador hasta su dosis, oirá un sonido de click y sentirá resistencia en el marcador por cada incremento para ayudarle a marcar la dosis correcta.
Limpieza
- La parte exterior de su pluma puede limpiarse con un paño humedecido en agua, si fuera necesario.
- No introduzca la pluma en agua ni en ningún otro líquido.
Almacenamiento
- Guarde siempre la pluma con la tapa puesta y sin aguja fijada.
- No use la pluma después de la fecha de de caducidad (CAD) impreso en la etiqueta de la pluma.
- No conserve la pluma a temperaturas extremas, luz solar directa o condiones muy frías, como un coche o un congelador.
- Conserve la pluma fuera del alcance de los niños y de cualquier persona que no haya sido entrenada en el uso de la pluma.
Antes del uso:
- Conserve la pluma en el frigorífico entre 2 °C y 8 °C. No congelar.
- Si se conserva fuera del frigorífico (a una temperatura igual o inferior a 25 °C), la pluma puede aguantar hasta 3 meses, incluyendo el periodo de uso. Deseche la pluma si no se ha utilizado después de 3 meses.
Después del primer uso (periodo de uso):
- La pluma puede almacenarse hasta 28 días a una temperatura igual o inferior a 25°C. No congelar.
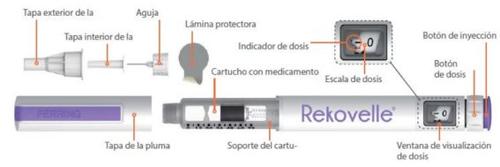
Artículos que necesitará para administrarse la inyección de REKOVELLE
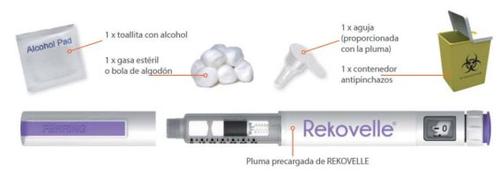
Antes de su uso – (Paso 1)
Paso 1:
- Lávese las manos.
- Compruebe la pluma para ver que no está dañada. No use la pluma si está dañada.
- Compruebe la pluma (cartucho) para ver que el medicamento está transparente y no contiene partículas. No use una pluma que contenga en el cartucho medicamento con partículas o turbio.
- Asegúrese de que tiene la pluma correcta con la concentración correcta.
- Compruebe la fecha de caducidad de la etiqueta de la pluma.
Fijación la aguja – (Pasos 2 a 6)
Importante:
- Use siempre una aguja nueva para cada inyección.
- Use sólo las agujas click-on de un sólo uso que se proporcionan con la pluma.
Paso 2:
- Retire la tapa de la pluma.
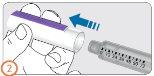
Paso 3:
- Retire la lámina protectora de la aguja.
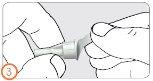
Paso 4:
- Encaje la aguja.
- Oirá o sentirá un “click” cuando la aguja esté encajada de forma segura.
- También podrá enroscar la aguja. Cuando sienta una ligera resistencia, estará encajada de forma segura.

Paso 5:
- Retire la tapa protectora externa de la aguja.
- No deseche la tapa externa de la aguja. La necesitará para desechar la aguja tras la inyección del medicamento.
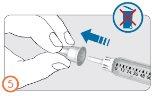
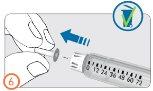
Paso 6:
- Retire la tapa interior de la aguja y deséchela.
Preparación – (Pasos 7 a 9)
- Antes de usar la pluma por primera vez, deberá eliminar las burbujas de aire del cartucho (cebado) para recibir la dosis correcta de medicamento.
- Sólo debe preparar la pluma la primera vez que la use.
- Realice los pasos 7 a 9 incluso si no observa burbujas de aire.
- Si la pluma ya ha sido utilizada, vaya directamente al paso 10.
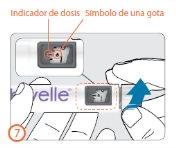
Paso 7:
- Gire el botón de dosis en el sentido de las agujas del reloj hasta que el símbolo de una gota se alinee con el indicador de dosis.
- Si marca la dosis incorrecta de preparación, puede ser corregida tanto hacia arriba como hacia abajo, sin pérdida de medicamento, girando el botón de dosis en cualquier dirección hasta que el símbolo de una gota se alinee con el indicador de dosis.
Paso 8:
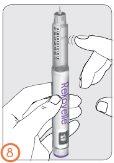
- Sujete la pluma con la aguja apuntando hacia arriba.
- Golpee con el dedo en el soporte del cartucho para conseguir que cualquier burbuja presente en el cartucho ascienda hasta la parte superior del cartucho.
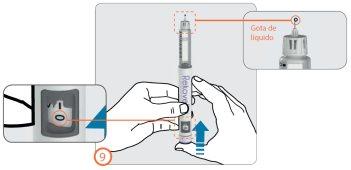
Paso 9:
- Con la aguja todavía apuntando hacia arriba (lejos de su cara) presione el botón de inyección hasta que vea el número ‘0’ en línea con el indicador de dosis.
- Compruebe que aparece una gota de líquido en la punta de la aguja.
- Si no aparece(n) la(s) gota(s) repita los pasos 7 a 9 (Preparación) hasta que aparezca una gota.
- Si no aparece una gota tras 5 intentos, retire la aguja (ver paso 13), y fije una nueva aguja
(ver pasos 3 a 6), y repita la preparación (ver pasos 7 a 9).
- • Si aún no ve una gota después de usar una aguja nueva, pruebe con una pluma nueva.
Marcado de la dosis – (Paso 10)
Ver “Ejemplos de como marcar una dosis” en las páginas 20 a 211.
Paso 10:
- Gire el botón de dosis en el sentido de las agujas del reloj hasta que la dosis prescrita se alinee con el indicador de dosis en la ventana de visualización de dosis.
- La dosis puede corregirse tanto hacia arriba como hacia abajo sin pérdida de medicamento, girando el botón de dosis en cualquier dirección hasta que la dosis correcta se alinee con el indicador de dosis.
- No presione el botón de inyección cuando esté marcando una dosis para evitar la pérdida de medicamento.

División de dosis:
- Puede necesitar más de una pluma para completar la dosis que se le ha prescrito.
- Si no logra marcar su dosis completa, esto quiere decir que no queda suficiente medicamento en la pluma. Necesitará administrarse la dosis dividiéndola en dos inyecciones o desechar su pluma y usar una nueva para su inyección.
Ver “Dar una dosis dividida de REKOVELLE“ en las páginas 22 a 231 para ejemplos de cómo calcular y registrar su dosis dividida.
Inyección de la dosis – (Pasos 11 a 12)
Importante:
- No use la pluma si el medicamento contiene partículas o está turbio.
- Lea los pasos 11 y 12 en las páginas 14 a 151 antes de administrarse su inyección.
- Este medicamento debe administrarse por inyección justo debajo de la piel (subcutánea) en el área del estómago (abdomen).
- Use un lugar de inyección nuevo para cada inyección para disminuir el riesgo de reacciones en la piel como enrojecimiento e irritación.
- No se inyecte en un área que esté irritada (sensible), con moretones, enrojecida, dura, con cicatrices o donde tenga estrías.
Pasos 11 y 12:
- Limpie la piel del lugar en el que vaya a inyectarse con un hisopo con alcohol para limpiarla. No vuelva a tocar este área antes de administrarse la inyección.
- Sujete la pluma de tal manera que la ventana de visualización de dosis sea visible durante la inyección.
- Pellizque su piel e inserte la aguja directamente como le haya enseñado su profesional sanitario. No toque el botón de inyección aún.
- Después de la inserción de la aguja, coloque su dedo pulgar en el botón de inyección.
- Presione el botón de inyección hasta el final y manténgalo apretado.
- Continúe apretando el botón de inyección y cuando vea el número ‘0’ en línea con el indicador de dosis, espere 5 segundos (contando despacio hasta 5). Esto asegurará que reciba su dosis completa.
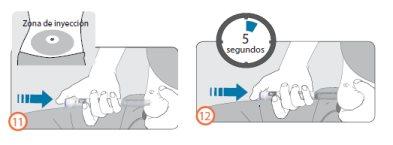
- Después de presionar el botón de inyección durante 5 segundos, suéltelo. Después retire la aguja lentamente del sitio de inyección, tirando de ella directamente fuera de la piel.
- Si aparece sangre en el sitio de inyección, presione ligeramente en el lugar con una gasa o una bola de algodón.
Nota:
- No incline la pluma ni durante la inyección ni durante la retirada de la aguja de la piel.
- La inclinación de la pluma puede producir que la aguja se doble o se rompa.
- Si una aguja rota se queda atascada en el cuerpo o debajo de la piel, busque ayuda sanitaria de inmediato.
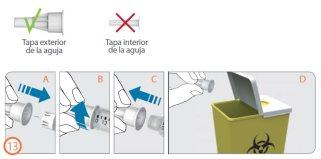
Desechar la aguja – (Paso 13)
Paso 13:
- Vuelva a colocar la tapa exterior de la aguja de forma cuidadosa con un empujón firme (A).
- Desenrolle la aguja en dirección contraria a las agujas del reloj para retirarla de la pluma (B+C).
- Deseche cuidadosamente la aguja utilizada (D).
- Ver “Eliminación” en la página 181.
Nota:
- Retire la aguja siempre después de cada uso. Las agujas son para un solo uso.
- No almacene la pluma con la aguja insertada.
Recolocación de la tapa en la pluma – (Paso 14)
Paso 14:
- Vuelva a colocar la tapa de la pluma firmemente para protegerla entre inyecciones.
Nota:
- La tapa de la pluma no encajará si hay una aguja fijada.
- Si se va a administrar una dosis dividida en dos inyecciones, deseche la pluma sólo cuando esté vacía.
- Si va a usar una nueva pluma para administrarse la dosis completa que se le ha prescrito en lugar de administrarse la dosis dividida en dos inyecciones, deseche la pluma cuando no quede suficiente medicamento para una dosis completa.
- Mantenga la tapa de la pluma puesta cuando no se esté usando.
Eliminación
Agujas:
Ponga las agujas usadas en un contenedor antipinchazos, como por ejemplo un contenedor para objetos punzantes, inmediatamente después del uso. No deseche el contenedor usado con su basura doméstica.
Si no tiene un contenedor para objetos punzantes, puede usar un contenedor doméstico que tenga las siguientes características:
- Que sea de plástico sólido y resistente,
- que pueda ser cerrado con una tapa ajustada y resistente a material punzante, sin que el material punzante pueda salir al exterior,
- que pueda permanecer en vertical y estable durante el uso,
- que sea a prueba de fugas, y
- que esté correctamente etiquetado para advertir que contiene residuos peligrosos.
Plumas precargadas de REKOVELLE:
- Deseche las plumas usadas de acuerdo con la legislación local de eliminación de basuras.
Ejemplos de cómo marcar una dosis
Ejemplos de cómo marcar una dosis usando su pluma precargada de REKOVELLE
La siguiente tabla muestra ejemplos de dosis prescritas, cómo marcar los ejemplos de dosis prescritas y cuál es el aspecto de la ventana de visualización de dosis para las dosis prescritas.
Ejemplos de dosis prescritas (en microgramos) | Dosis a marcar en la pluma | Ventana de visualización de dosis para los ejemplos de dosis prescrita |
0.33 | 0 y 1 línea (marque hasta 0 más 1 click) |
|
0.66 (dosis de preparación) | 0 y 2 líneas (marque hasta 0 más 2 clicks) |
|
2.33 | 2 y 1 línea (marque hasta 2 más 1 click) |
|
11.00 | 11 (marque hasta 11) |
|
12.33 | 12 y 1 línea (marque hasta 12 más 1 click) |
|
18.66 | 18 y 2 líneas (marque hasta 18 más 2 clicks) |
|
20.00 | 20 (marque hasta 20) |
|
Administración de una dosis dividida de REKOVELLE
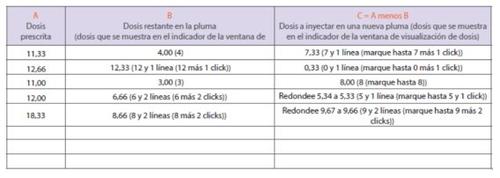
Si no logra marcar la dosis completa que se le ha prescrito en su pluma, esto quiere decir que no queda suficiente medicamento en la pluma para administrar la dosis completa. Deberá administrarse parte de su dosis prescrita utilizando la pluma que estaba usando, y el resto de la dosis utilizando una nueva pluma (inyección de dosis dividida) o deberá desechar la pluma que estaba usando y usar una pluma nueva para administrarse la dosis completa que se le ha prescrito en una sóla inyección. Si decide administrarse la dosis dividida en dos inyecciones, siga estas instrucciones, y escriba la cantidad de medicamento que se debe administrar usando el diario de dosis dividida de la página 231.
- La columna A muestra un ejemplo de una dosis prescrita. Escriba la dosis que se le ha prescrito en la columna A.
- La columnaB muestra un ejemplo de la dosis que queda en la pluma (esta es igual a la dosis que sea capaz de marcar).
- Escriba la dosis que queda en su pluma en la columna B. Adminístrese la inyección usando la cantidad de medicamento restante que queda en su pluma.
- Prepare una nueva pluma (pasos 1 a 9).
- Calcule y escriba la dosis restante que se debe inyectar en la columna C, para esto debe restar el número en la columna B al número en la columna A. Use una calculadora para comprobar que ha hecho bien la operación, si fuera necesario.
- Vea “Ejemplos de cómo marcar una dosis” en las páginas 20 a 211 si fuera necesario.
- Las dosis se deben redondear al incremento más cercano, X.00, X.33 o X.66 microgramos. Por ejemplo, si el número en la columna C es 5.34, redondee su dosis a 5.33. si el número en la columna C es C es 9.67, redondee su dosis a 9.66.
- Contacte con su profesional sanitario si tiene dudas acerca de cómo calcular su dosis dividida.
- Inyéctese la dosis restante de medicamento (el número en la columna C) usando una nueva pluma para completar la dosis prescrita.
Diario de dosis dividida
Preguntas frecuentes
- ¿El paso de preparación es necesario antes de cada inyección?
- No. la preparación debe realizarse sólo antes de la administración de la primera inyección con una nueva pluma.
- ¿Cómo puedo saber si la inyección está completa?
- El botón de inyección se ha presionado firmemente hasta el final hasta que se ha parado.
- El número ‘0’ se encuentra en línea con el indicador de dosis.
- Ha contado lentamente hasta 5 mientras está sujetando el botón de inyección y la aguja está inyectada aún en la piel.
- ¿Porqué se debe contar hasta 5 mientras se sujeta el botón de inyección?
- Sujetar el botón de inyección durante 5 segundos permite que la dosis completa se inyecte y se absorba debajo de su piel.
- ¿Qué sucede si el botón de dosis no puede girarse hasta la dosis prescrita?
- Es posible que al cartucho de la pluma no le quede suficiente medicamento para liberar la dosis prescrita.
- La pluma no le permite marcar una dosis mayor de la que queda en el cartucho.
- Puede inyectarse la cantidad de medicamento que quede en la pluma y completar la dosis prescrita con una nueva pluma (dosis dividida) o usar una nueva pluma para administrarse la dosis completa prescrita.
Precauciones
- No use una pluma que se haya caído o golpeado contra superficies duras.
- Si no es fácil pulsar el botón de inyección, no utilice la fuerza. Cambie la aguja. Si el botón de inyección continúa siendo difícil de pulsar después de cambiar la aguja, use una nueva pluma.
- No intente arreglar una pluma dañada. Si una pluma está dañada, contacte con su profesional sanitario o representante local del titular de la autorización de comercialización (consulte el prospecto para la información de contacto).
Información adicional
Agujas
Las agujas se proporcionan con la pluma. Si necesitara agujas adicionales contacte con su profesional sanitario. Use sólo las agujas que acompañan a la pluma precargada de REKOVELLE o las agujas que le prescriba su profesional sanitario.
Contacto
Si tiene cualquier pregunta o problema en relación con la pluma, contacte con su profesional sanitario o representante local del titular de la autorización de comercialización (consulte el prospecto para la información de contacto).
- Los números de página se refieren al manual de Instrucciones de Uso impreso y no a los números de página de este documento.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REKOVELLE 36 MICROGRAMOS/1,08 ML SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 12 microgramos/0,36 mlPrincipio activo: Follitropin deltaFabricante: Ferring Pharmaceuticals A/SRequiere recetaForma farmacéutica: INYECTABLE, 72 µgPrincipio activo: Follitropin deltaFabricante: Ferring Pharmaceuticals A/SRequiere recetaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para REKOVELLE 36 MICROGRAMOS/1,08 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REKOVELLE 36 MICROGRAMOS/1,08 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes













