REKAMBYS 900 MG SUSPENSION INYECTABLE DE LIBERACION PROLONGADA

Cómo usar REKAMBYS 900 MG SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
REKAMBYS 900 mg suspensión inyectable de liberación prolongada
rilpivirina
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es REKAMBYS y para qué se utiliza
- Qué necesita saber antes de empezar a usar REKAMBYS
- Cómo se administra REKAMBYS
- Posibles efectos adversos
- Conservación de REKAMBYS
- Contenido del envase e información adicional
1. Qué es REKAMBYS y para qué se utiliza
REKAMBYS contiene el principio activo rilpivirina. Forma parte de un grupo de medicamentos denominados inhibidores de la transcriptasa inversa no análogos de nucleósidos (ITINAN) que se emplean en el tratamiento del virus de la inmunodeficiencia humana tipo 1 (VIH-1).
REKAMBYS actúa junto con otros medicamentos contra el VIH bloqueando la capacidad del virus para hacer más copias de sí mismo. REKAMBYS inyectable no cura la infección por el VIH, pero ayuda a reducir la cantidad de VIH en su cuerpo y la mantiene a un nivel bajo. Así frena el daño al sistema inmunitario y el desarrollo de infecciones y enfermedades asociadas al SIDA.
REKAMBYS siempre se administra con otro medicamento contra el VIH que se llama cabotegravir inyectable. Se administran juntos en adultos a partir de los 18 años cuya infección por el VIH-1 ya está controlada.
2. Qué necesita saber antes de empezar a usar REKAMBYS
No use REKAMBYSsi es alérgico a rilpivirina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
No use REKAMBYS si está tomando alguno de los medicamentos siguientes, ya que pueden afectar a la forma en la que REKAMBYS o los otros medicamentos actúan:
- carbamazepina, oxcarbazepina, fenobarbital, fenitoína (medicamentos para tratar la epilepsia y prevenir las convulsiones)
- rifabutina, rifampicina, rifapentina (medicamentos para tratar infecciones bacterianas como la tuberculosis)
- dexametasona (un corticosteroide que se emplea para tratar diversas patologías, tales como la inflamación y las reacciones alérgicas) administrada en un ciclo de tratamiento por vía oral o inyectable
- productos que contienen Hierba de San Juan o Hipérico (Hypericum perforatum,una planta medicinal que se emplea para la depresión).
Si está tomando alguno de los medicamentos anteriores, consulte a su médico sobre las alternativas.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar REKAMBYS.
REKAMBYS no cura la infección por el VIH. Forma parte de un tratamiento para reducir la cantidad de virus en la sangre. Mientras esté usando este medicamento aún puede transmitir el VIH a los demás, aunque el riesgo se reduce por el tratamiento antiviral eficaz. Consulte a su médico sobre las precauciones que debe tomar para no infectar a otras personas.
Informe a su médico sobre su situación
Revise los siguientes puntos e informe a su médico si se encuentra en alguno de los siguientes casos.
- Debe acudir a todas las visitas programadas para las inyecciones, no falte a ninguna visita, es muy importante para el éxito de su tratamiento. Si no puede acudir a una visita programada, informe a su médico lo antes posible.
- Informe a su médico si tiene o ha tenido alguna vez enfermedades del hígado, incluidas la hepatitis B o la hepatitis C, o enfermedades del riñón. Su médico probablemente compruebe cómo de bien funcionan su hígado y riñones para decidir si puede usar REKAMBYS. Consulte los signos de daño hepático en la sección 4 de este prospecto “Efectos adversos poco frecuentes”.
- Informe a su médico inmediatamente si observa algún síntoma de infección(por ejemplo, fiebre, escalofríos, sudores). En algunos pacientes con VIH, puede ocurrir inflamación debido a infecciones previas poco después de iniciar el tratamiento contra el VIH. Se cree que estos síntomas se deben a la mejoría de la respuesta inmunitaria del organismo, lo que le permite combatir las infecciones presentes anteriormente pero que no habían manifestado síntomas evidentes.
- Informe inmediatamente a su médico si observa cualquier síntoma como por ejemplo debilidad muscular, debilidad que empieza en manos y pies y que asciende hacia el tronco del cuerpo, palpitaciones, temblor o hiperactividad. Esto se debe a trastornos autoinmunes (condiciones en las que el sistema inmunitario ataca por error tejido corporal sano) que también pueden ocurrir después de que usted haya empezado a tomar medicamentos para el tratamiento de su infección por VIH. Los trastornos autoinmunes pueden aparecer muchos meses después del inicio del tratamiento.
- Informe a su médico si está tomando algún medicamento que le hayan dicho que puede causar un latido irregular del corazón potencialmente mortal (torsade de pointes).
Reacciones a las inyecciones
Algunas personas han experimentado síntomas de reacciones posteriores a la inyección a los pocos minutos de recibir la inyección de rilpivirina. La mayoría de los síntomas se resolvieron unos minutos después de la inyección. Los síntomas de las reacciones posteriores a la inyección pueden incluir: dificultad para respirar, calambres estomacales, sudoración, entumecimiento de la boca, sensación de ansiedad, sensación de calor, sensación de mareo o de que se va a desvanecer (o a desmayar) y cambios en la presión arterial. Informe a su profesional sanitario si experimenta estos síntomas después de recibir sus inyecciones.
Es importante acudir puntualmente a las citas
Es importante que acuda a sus citas programadaspara recibir REKAMBYS para controlar su infección por el VIH y para evitar que la enfermedad empeore. No falte a ninguna de las visitas, es muy importante para que el tratamiento sea eficaz. Si no puede acudir a una visita programada, informe a su médico lo antes posible. Informe a su médico si está pensando dejar el tratamiento. Si la administración de REKAMBYS se retrasa, o si deja de recibir REKAMBYS, deberá tomar otros medicamentos para tratar la infección por el VIH y reducir el riesgo de que el virus se haga resistente, ya que los niveles de medicamento en su cuerpo serán demasiado bajos para tratar la infección por el VIH.
Niños
REKAMBYS no es para uso en niños ni adolescentes menores de 18 años de edad, ya que no se ha estudiado en estos pacientes.
Otros medicamentos y REKAMBYS
Informe a su profesional sanitario si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Algunos medicamentos pueden afectar al nivel de REKAMBYS en sangre si los toma durante el tratamiento con REKAMBYS, o REKAMBYS puede afectar a la eficacia de los otros medicamentos.
No use REKAMBYS si está tomando alguno de los medicamentos siguientes, ya que pueden afectar a la forma en la que REKAMBYS o los otros medicamentos actúan:
- carbamazepina, oxcarbazepina, fenobarbital, fenitoína (medicamentos para tratar la epilepsia y prevenir las convulsiones)
- rifabutina, rifampicina, rifapentina (medicamentos para tratar infecciones bacterianas como la tuberculosis)
- dexametasona (un corticosteroide que se emplea para tratar diversas patologías, tales como la inflamación y las reacciones alérgicas) administrada en un ciclo de tratamiento por vía oral o inyectable
- productos que contienen Hierba de San Juan o Hipérico (Hypericum perforatum,una planta medicinal que se emplea para la depresión).
Si está tomando alguno de los medicamentos anteriores, consulte a su médico sobre las alternativas.
El efecto de REKAMBYS o de otros medicamentos puede cambiarsi usa REKAMBYS con cualquiera de los siguientes medicamentos:
- claritromicina, eritromicina (antibióticos)
- metadona (que se emplea para tratar el síndrome de abstinencia y la dependencia)
Embarazo y lactancia
Informe a su médico inmediatamente si está embarazada o tiene intención de quedarse embarazada. Su médico valorará el beneficio y el riesgo, para usted y su bebé, del uso de REKAMBYS durante el embarazo. Si tiene intención de quedarse embarazada, consulte a su médico previamente, ya que rilpivirina puede permanecer en el organismo hasta 4 años después de la última inyección de REKAMBYS.
Las mujeres que tienen VIH no deben dar el pecho porque el VIH se puede trasmitir a través de la leche materna e infectar al bebé.
Consulte a su médico o farmacéutico antes de tomar cualquier medicamento.
Conducción y uso de máquinas
Algunos pacientes pueden sentir cansancio, mareo o somnolencia durante el tratamiento con REKAMBYS. No conduzca ni utilice máquinas si sufre cualquiera de estos efectos adversos.
Información importante sobre algunos de los componentes de REKAMBYS
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 3 ml de injeccion; esto es, esencialmente “exento de sodio”.
3. Cómo se administra REKAMBYS
Un enfermero o médico le administrará REKAMBYS como una inyección en el músculo de la nalga (inyección intramuscular, o IM).
La inyección se le administrará una vez al mes o una vez cada 2 meses,junto con otro medicamento inyectable que se llama cabotegravir. Su médico le explicará con qué frecuencia se le administrará el medicamento.
Antes de comenzar el tratamiento con REKAMBYS, su médico le prescribirá un tratamiento diario con comprimidos de rilpivirina y cabotegravir durante un mes. Esto se conoce como dosificacióninicial oral;tomando los comprimidos antes de recibir las inyecciones de RECAMBYS y cabotegravir permitirá a su médico comprobar si éstos medicamentos son adecuados para usted.
Si le van a administrar REKAMBYS una vez al mes, su tratamiento será el siguiente:
Cuándo | |||
Medicamento | Mes 1 (al menos 28 días) | Mes 2 (después de un mes de comprimidos) | A partir del mes 3 |
Rilpivirina | Comprimido de 25 mg una vez al día | Una inyección de 900 mg | Inyección de 600 mg cada mes |
Cabotegravir | Comprimido de 30 mg una vez al día | Una inyección de 600 mg | Inyección de 400 mg cada mes |
Si le van a administrar REKAMBYS cada 2 meses, su tratamiento será el siguiente:
Cuándo | |||
Medicamento | Mes 1 (al menos 28 días) | Mes 2 (después de un mes de comprimidos) y mes 3 | A partir del mes 5 |
Rilpivirina | Comprimido de 25 mg, una vez al día | Una inyección de 900 mg | Inyección de 900 mg cada 2 meses |
Cabotegravir | Comprimido de 30 mg, una vez al día | Una inyección de 600 mg | Inyección de 600 mg cada 2 meses |
Si omite una inyección de REKAMBYS
Es importante que acuda regularmente a sus citas programadas para recibir su inyección. Si no acude a una cita, póngase en contacto con su médico inmediatamente para concertar otra.
Informe a su médicosi cree que no va a poder recibir la inyección de REKAMBYS en la fecha prevista. Su médico le puede recomendar que tome comprimidos hasta que le puedan administrar una inyección de REKAMBYS de nuevo.
Si le administran demasiado REKAMBYS
Un médico o enfermero le administrará este medicamento, por lo que es improbable que le administren demasiado. Si está preocupado, dígaselo al médico o enfermero.
No deje de usar REKAMBYS sin que se lo recomiende su médico.
Use REKAMBYS todo el tiempo que se lo indique su médico. No interrumpa el tratamiento a no ser que su médico se lo aconseje.
Después de interrumpir el tratamiento pueden quedar niveles bajos de rilpivirina (el principio activo de REKAMBYS) en su cuerpo durante hasta 4 años. No obstante, después de que le administren la última inyección de REKAMBYS, los niveles bajos de rilpivirina que queden no serán suficiente para luchar contra el virus y se podría hacer resistente. Para mantener la infección de VIH-1 bajo control y evitar que el virus se haga resistente, debe empezar un tratamiento diferente contra el VIH en la fecha en que estuviera prevista la siguiente inyección de REKAMBYS.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
A continuación, encontrará una lista de los efectos adversos que se han descrito con el uso de REKAMBYS y cabotegravir inyectable.
Efectos adversos muy frecuentes (afectan al menos a 1 de cada 10 personas)
- dolor de cabeza
- reacciones en el lugar de la inyección - habitualmente son de leves a moderadas y su frecuencia disminuye con el tiempo. Sus síntomas pueden incluir:
o muy frecuentes: dolor y molestias, masas o bultos duros
o frecuentes: enrojecimiento, picor, hinchazón, hematomas, calor o cambio de color.
o poco frecuentes: entumecimiento, sangrado leve, formación de absceso (acumulación de pus) o celulitis (con sensación de calor, hinchazón o enrojecimiento).
- Sensación de calor/febril (pirexia)
Efectos adversos frecuentes (afectan a menos de 1 de cada 10 personas)
- depresión
- ansiedad
- sueños anormales
- dificultad para dormir (insomnio)
- mareos
- sensación de malestar (náuseas)
- vómitos
- dolor de tripa (dolor abdominal)
- gases (flatulencia)
- diarrea
- sarpullido
- dolor muscular (mialgia)
- cansancio (fatiga)
- sensación de debilidad (astenia)
- malestar general
- aumento de peso
Efectos adversos poco frecuentes (afectan a menos de 1 de cada 100 personas)
- adormecimiento (somnolencia)
- sensación de mareo durante o después de una inyección. Esto puede provocar desvanecimientos.
- daño hepático (sus signos pueden incluir coloración amarilla de la piel y la parte blanca del ojo, pérdida del apetito, picor, dolor a la palpación de la tripa, heces de color claro u orina de un color anormalmente oscuro).
- cambios en los niveles analíticos de función hepática (aumento de las transaminasas)
- aumento de la bilirrubina(una sustancia producida por el hígado) en sangre.
Otros efectos adversos
- Dolor abdominal intenso causado por inflamación del páncreas (pancreatitis).
Los siguientes efectos adversos que se pueden producir con rilpivirina en comprimidos también se pueden dar con REKAMBYS inyectable:
Efectos adversos muy frecuentes
- aumento del colesterol y / o amilasa pancreática en sangre
Efectos adversos frecuentes (afectan a menos de 1 de cada 10 personas)
- pérdida del apetito
- trastornos del sueño
- estado depresivo
- molestias de estómago
- sequedad en la boca
- recuento bajo de glóbulos blancos y / o plaquetas, disminución de la hemoglobina en sangre, aumento de triglicéridos y / o lipasa en sangre
Efectos adversos poco frecuentes (afectan a menos de 1 de cada 100 personas)
- signos o síntomas de inflamación o infección, por ejemplo fiebre, escalofríos, sudoración (síndrome de reconstitución inmune, para más información ver la sección 2)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de REKAMBYS
Mantener fuera de la vista y del alcance de los niños.
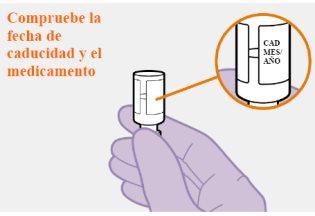
No use este medicamento después de la fecha de caducidad que encontrará en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8°C). No congelar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de REKAMBYS
- El principio activo es rilpivirina. Cada vial de 3 ml contiene 900 mg de rilpivirina.
- Los excipientes son poloxámero 338, ácido cítrico monohidratado, glucosa monohidrato, dihidrogenofosfato de sodio monohidratado, hidróxido de sodio para ajuste del pH y garantizar la isotonicidad, agua para inyectables.
Aspecto del producto y contenido del envase
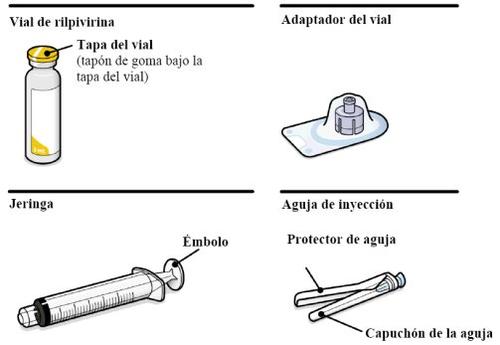
Suspensión inyectable de liberación prolongada. REKAMBYS se presenta en un vial de vidrio. El envase también contiene 1 jeringa, 1 adaptador del vial y 1 aguja de inyección.
Titular de la autorización de comercialización
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Responsable la fabricación
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien ViiV Healthcare srl/bv Tél/Tel: + 32 (0) 10 85 65 00 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
???????? „??????? & ??????? ????????” ???? ???.: +359 2 489 94 00 | Luxembourg/Luxemburg ViiV Healthcare srl/bv Belgique/Belgien Tél/Tel: + 32 (0) 10 85 65 00 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD. Tel: +356 2397 6000 |
Deutschland ViiV Healthcare GmbH Tel.: + 49 (0)89 203 0038-10 | Nederland ViiV Healthcare BV Tel: + 31 (0) 33 2081199 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλ?δα Janssen-Cilag Φαρμακευτικ? Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Laboratorios ViiV Healthcare, S.L. Tel: + 34 900 923 501 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France ViiV Healthcare SAS Tél.: + 33 (0)1 39 17 69 69 | Portugal VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Tel: + 351 21 094 08 01 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson s.r.o. Tel: +421 232 408 400 |
Italia ViiV Healthcare S.r.l Tel: +39 045 7741600 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Κ?προς Βαρν?βας Χατζηπαναγ?ς Λτδ Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom ViiV Healthcare UK Limited Tel: + 44 (0)800 221441 |
Fecha de la última revisión de este prospecto: {MM/AAAA}.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/.
Esta información está destinada únicamente a los médicos o profesionales sanitarios que deben leerla junto con la información de prescripción completa (Resumen de las Características del Producto).
Instrucciones de uso de REKAMBYS 3 ml inyectable:
Resumen Una dosis completa consta de dos inyecciones: 3 ml de cabotegravir y 3 ml de rilpivirina. Cabotegravir y rilpivirina se presentan en suspensiones que no requieren dilución o reconstitución. Los pasos para la preparación de ambos medicamentos son los mismos. Cabotegravir y rilpivirina son únicamente para administración intramuscular. Ambas inyecciones se deben administrar en el glúteo. El orden de la administración no es importante. Nota:Se recomienda administrar en la zona ventroglútea. | |
Información de conservación | |
Nocongelar. | |
| |
Su envase contiene | |
Tenga en cuenta la constitución del paciente y utilice su criterio médico para seleccionar la aguja de inyección de longitud adecuada. | |
También necesitará | |
| |
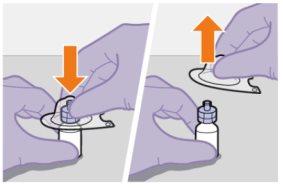
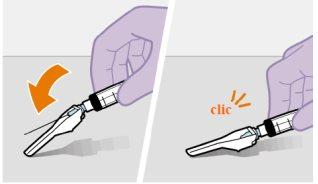
Preparación | |
| |
|
No usar sí la fecha de caducidad ha vencido. |
| |
|
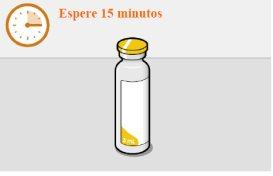
administrar la inyección para que el medicamento alcance temperatura ambiente |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
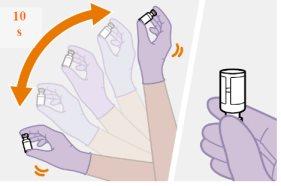
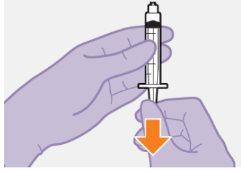
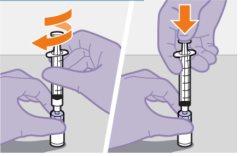
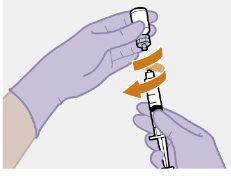
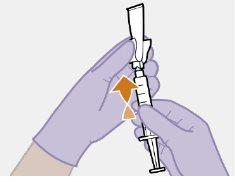
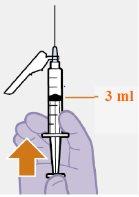
Nota: Mantenga la jeringa hacia arriba par evitar que gotee. Compruebe que la suspensión tiene un aspecto uniforme y color blanco lechoso. |
| |
|
|
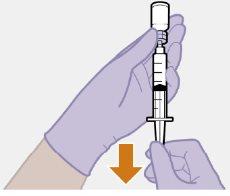
Inyección | |
| |
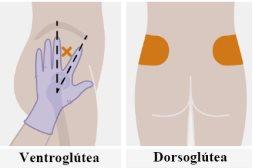
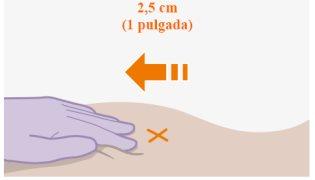
| Las inyecciones se deben administrar en el glúteo. Seleccione una de las siguientes zonas para la inyección:
Nota: Solo para administración por inyección intramuscular en el glúteo. Noadministrar por vía intravenosa. |
| |
|
|
| |
|
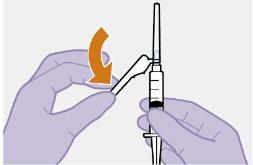
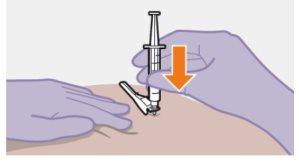
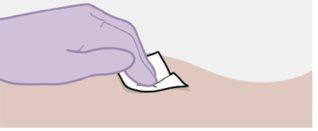
Nota: Limpie la zona de la inyección con una toallita impregnada en alcohol. Deje que la piel se seque al aire antes de continuar. |
| |
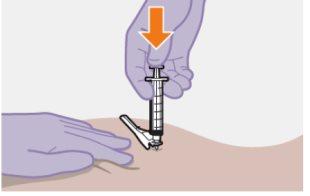
| Use la técnica de inyección en z para minimizar en lo posible que el medicamento escape del lugar de inyección.
|
| |
|
|
| |
|
|
| |
|
Nomasajee la zona. |
| |
|
|
Después de la inyección | |
| |
|
|
Repita el proceso para el 2º medicamento | |
| Si aún no ha inyectado los dos medicamentos, siga los pasos para la preparación e inyección de cabotegravir, que tiene sus propias Instrucciones de uso. |
Preguntas y respuestas | |
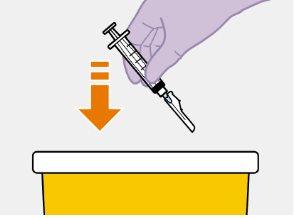
Es mejor inyectar el medicamento en cuanto alcance temperatura ambiente. No obstante, el vial se puede dejar en su envase a temperatura ambiente (temperatura máxima de 25°C) durante un máximo de 6 horas.
Es mejor inyectar el medicamento (a temperatura ambiente) lo antes posible después de extraerlo del vial. No obstante, el medicamento se puede dejar en la jeringa durante un máximo de 2 horas antes de inyectarlo. Si se superan las 2 horas, desechar el medicamento, la jeringa y la aguja.
Inyectar 1 mL de aire al vial facilita la extracción de la dosis con la jeringa. Si no se inyecta aire, parte del líquido puede volver a entrar en el vial de forma accidental, dejando una cantidad insuficiente en la jeringa.
No, el orden no es importante.
Es mejor dejar que el vial alcance temperatura ambiente de forma natural. No obstante, se puede utilizar el calor de las manos para acelerar el proceso, pero asegúrese de que el vial no supera los 25°C No utilice ningún otro método para calentarlo.
Se recomienda la administración en la zona ventroglútea, en el músculo glúteo medio, porque es una zona en la que no hay nervios ni vasos importantes cerca. Si el profesional sanitario lo prefiere, también es aceptable la administración en la zona dorsoglútea, en el músculo glúteo mayor. La inyección no se debe administrar en ninguna otra zona. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REKAMBYS 900 MG SUSPENSION INYECTABLE DE LIBERACION PROLONGADAForma farmacéutica: COMPRIMIDO, 25 mg rilpivirinaPrincipio activo: RilpivirinaFabricante: Janssen-Cilag International N.VRequiere recetaForma farmacéutica: COMPRIMIDO, 600 mgPrincipio activo: EfavirenzFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para REKAMBYS 900 MG SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REKAMBYS 900 MG SUSPENSION INYECTABLE DE LIBERACION PROLONGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes