
RAPAMUNE 1 mg/ml SOLUCION ORAL

Cómo usar RAPAMUNE 1 mg/ml SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Rapamune 1 mg/ml solución oral
sirolimus
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
|
Contenido del prospecto
- Qué es Rapamune y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Rapamune
- Cómo tomar Rapamune
- Posibles efectos adversos
- Conservación de Rapamune
- Contenido del envase e información adicional
1. Qué es Rapamune y para qué se utiliza
Rapamune contiene el principio activo sirolimus, que pertenece al grupo de medicamentos denominados inmunosupresores. Le ayuda a controlar su sistema inmune corporal después de haber recibido un trasplante de riñón.
Rapamune se usa en adultos para impedir el rechazo de los riñones trasplantados y normalmente se usa junto con otros medicamentos inmunosupresores denominados corticosteroides, e inicialmente (los primeros 2 a 3 meses) con ciclosporina.
Rapamune también se usa para el tratamiento de pacientes con linfangioleiomiomatosis esporádica (S-LAM) con enfermedad pulmonar moderada o deterioro de la función pulmonar. La S-LAM es una enfermedad pulmonar progresiva rara que afecta principalmente a mujeres en edad fértil. El síntoma más frecuente de la S-LAM es la dificultad para respirar.
2. Qué necesita saber antes de empezar a tomar Rapamune
No tome Rapamune
- si es alérgico a sirolimus o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si es alérgico al cacahuete o a la soja.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Rapamune.
- Si tiene algún problema en el hígado o ha tenido alguna enfermedad que pueda haber afectado a su hígado, informe a su médico ya que esto puede determinar la dosis de Rapamune que recibe y puede ser motivo de que se le realicen otros análisis de sangre.
- Rapamune, como otros medicamentos inmunosupresores, puede reducir su capacidad para combatir las infecciones y puede aumentar el riesgo de desarrollar cáncer en los tejidos linfoides y en la piel.
- Si tiene un índice de masa corporal (IMC) por encima de 30 kg/m2, puede presentar un mayor riesgo de cicatrización anormal de las heridas.
- Si usted está considerado como paciente con alto riesgo de sufrir un rechazo renal, por ejemplo si se sometió a un trasplante previo que rechazó.
Su médico le realizará pruebas para controlar sus niveles de Rapamune en sangre. También le realizará pruebas para controlar la función del riñón, para medir sus niveles de lípidos (colesterol y/o triglicéridos) en sangre, y posiblemente la función hepática, durante el tratamiento con Rapamune.
La exposición a la luz solar y a la luz UV debe limitarse cubriéndose la piel con ropa y usando un protector solar con elevado factor de protección, debido al incremento del riesgo de padecer cáncer de piel.
Niños y adolescentes
La experiencia sobre el uso de Rapamune en niños y adolescentes menores de 18 años de edad es limitada. No se recomienda el uso de Rapamune en esta población.
Toma de Rapamune con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Algunos medicamentos pueden interferir con la acción de Rapamune y, por tanto, podría necesitar un ajuste de dosis. En particular, debe informar a su médico o farmacéutico si está tomando cualquiera de los siguientes medicamentos:
- cualquier otro medicamento inmunosupresor.
- antibióticos o medicamentos antifúngicos usados para tratar infecciones, por ejemplo, claritromicina, eritromicina, telitromicina, troleandomicina, rifabutina, clotrimazol, fluconazol e itraconazol. No se recomienda tomar Rapamune junto con rifampicina, ketoconazol o voriconazol.
- cualquier medicamento usado para la tensión alta o para problemas del corazón, incluyendo nicardipino, verapamilo y diltiazem.
- medicamentos antiepilépticos, incluyendo carbamazepina, fenobarbital y fenitoína.
- medicamentos utilizados para el tratamiento de úlceras u otros problemas gastrointestinales, tales como cisaprida, cimetidina o metoclopramida.
- bromocriptina (utilizada en el tratamiento de la enfermedad de Parkinson y varios trastornos hormonales), danazol (utilizado en el tratamiento de trastornos ginecológicos) o inhibidores de la proteasa (por ejemplo, para VIH y hepatitis C tales como ritonavir, indinavir, boceprevir y telaprevir).
- Hierba de San Juan (Hypericum perforatum).
- letermovir (un medicamento antiviral para prevenir enfermar por citomegalovirus).
- cannabidiol (su uso incluye, entre otros, el tratamiento de las crisis epilépticas).
Se debe evitar el uso de vacunas vivas durante el tratamiento con Rapamune. Antes de la vacunación, informe a su médico o farmacéutico de que está recibiendo Rapamune.
El uso de Rapamune puede conducir a un incremento de los niveles de colesterol y triglicéridos en sangre (grasas sanguíneas) que puede requerir tratamiento. Los medicamentos conocidos como “estatinas” y “fibratos” utilizados para tratar el colesterol y los triglicéridos elevados, se han asociado con un riesgo incrementado de rotura de fibra de los músculos (rabdomiolisis). Informe a su médico si está tomando medicamentos para reducir las grasas sanguíneas.
El uso combinado de Rapamune e inhibidores de la enzima convertidora de angiotensina (ECA) (un tipo de medicamentos utilizados para bajar la tensión arterial) puede provocar reacciones alérgicas. Informe a su médico si está tomando estos medicamentos.
Toma de Rapamune con alimentos y bebidas
Tome Rapamune siempre de la misma manera, con o sin comida. Si prefiere tomar Rapamune con alimentos, debe tomarlo siempre con ellos. Si prefiere tomar Rapamune sin alimentos, debe tomarlo siempre sin ellos. La comida puede alterar la cantidad de medicamento que entra en la sangre y, por tanto, al tomar su medicamento siempre de la misma manera, los niveles de Rapamune en sangre se mantienen más estables.
No tome Rapamune con zumo de pomelo.
Embarazo, lactancia y fertilidad
No debe utilizarse Rapamune durante el embarazo a menos que sea claramente necesario. Debe usar un método anticonceptivo eficaz durante el tratamiento con Rapamune y durante las 12 semanas siguientes a la interrupción del tratamiento. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se sabe si Rapamune pasa a la leche materna. Las pacientes que tomen Rapamune deben dejar la lactancia.
Se ha asociado una reducción del recuento de espermatozoides con el uso de Rapamune, que habitualmente vuelve a la normalidad después de suspender el tratamiento.
Conducción y uso de máquinas
Aunque no se espera que el tratamiento con Rapamune pueda afectar a su capacidad para conducir, si tiene alguna duda, consulte con su médico.
Rapamune contiene etanol (alcohol)
Rapamune contiene hasta un 3,17% de etanol (alcohol). Una dosis inicial de 6 mg contiene hasta 150 mg de alcohol, lo que equivale a 3,80 ml de cerveza o a 1,58 ml de vino. Esta cantidad de alcohol puede ser perjudicial para las personas que padecen alcoholismo, así como para las mujeres embarazadas o en periodo de lactancia, niños y grupos de alto riesgo, como pacientes con enfermedades del hígado o epilepsia. El alcohol puede modificar o aumentar el efecto de otros medicamentos.
Las dosis de mantenimiento de 4 mg o menores contienen pequeñas cantidades de etanol (100 mg o menores), cantidades probablemente demasiado bajas para ser dañinas.
3. Cómo tomar Rapamune
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico decidirá la dosis exacta de Rapamune que debe tomar y la frecuencia con la que debe hacerlo. Siga exactamente las instrucciones de su médico y nunca cambie la dosis por su cuenta.
Rapamune es únicamente para uso oral. Informe a su médico si tiene dificultades para tomar la solución oral.
Tome Rapamune siempre de la misma manera, con o sin comida.
Trasplante renal
Su médico le dará una dosis inicial de 6 mg tan pronto como sea posible después de la operación de trasplante renal. Después necesitará tomar 2 mg de Rapamune todos los días hasta que su médico le indique otra cosa. Su dosis será ajustada dependiendo del nivel de Rapamune en sangre. Su médico necesitará realizar pruebas sanguíneas para medir las concentraciones de Rapamune.
Si también está tomando ciclosporina, tiene que espaciar la toma de los dos medicamentos aproximadamente 4 horas.
Se recomienda utilizar primero Rapamune en combinación con ciclosporina y corticosteroides. Al cabo de 3 meses, su médico puede suspender Rapamune o ciclosporina, ya que no se recomienda tomar juntos estos medicamentos pasado este tiempo.
Linfangioleiomiomatosis esporádica (S-LAM)
Su médico le dará 2 mg de Rapamune al día, hasta que le indique lo contrario. Su dosis se ajustará según el nivel de Rapamune en su sangre. Su médico deberá realizar análisis de sangre para medir las concentraciones de Rapamune.
Instrucciones sobre cómo diluir Rapamune
- Retire la tapa de seguridad del frasco presionando las lengüetas de la tapa y girando. Inserte el adaptador de jeringa en el frasco hasta que esté al nivel de la parte superior del frasco. No intente sacar el adaptador de la jeringa del frasco una vez insertado.
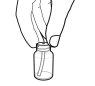
- Con el émbolo totalmente presionado, inserte una de las jeringas de dosificación en la abertura del adaptador.
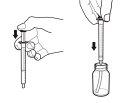
- Extraiga la cantidad exacta de solución oral de Rapamune que le haya recetado su médico tirando suavemente del émbolo de la jeringa de dosificación hasta que el nivel de la solución oral esté igualado con la marca apropiada de la jeringa de dosificación. El frasco debe permanecer en posición vertical mientras se está extrayendo la solución. Si se forman burbujas en la solución oral en la jeringa de dosificación durante la extracción, vuelva a introducir la solución de Rapamune en el frasco y repita el procedimiento de extracción. Puede que tenga que repetir el paso 3 más de una vez para obtener su dosis.
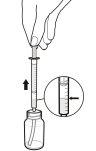
- Puede que su médico le haya indicado que tome la solución oral de Rapamune en un momento concreto del día. Si necesita llevar consigo el medicamento, llene la jeringa de dosificación hasta la marca apropiada y coloque la tapa de forma segura – la tapa debe quedar fija en su sitio. Después ponga la jeringa de dosificación tapada en la caja que se proporciona para el transporte. Una vez en la jeringa, el medicamento puede mantenerse a temperatura ambiente (sin exceder los 25ºC) o refrigerarse y debe usarse dentro de un periodo de 24 horas.
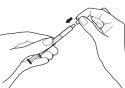
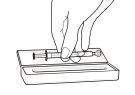
- Vacíe el contenido de la jeringa dosificadora en un vaso de vidrio o plástico con, al menos, 60 ml de agua o de zumo de naranja. Agite bien durante un minuto y bébaselo inmediatamente de una vez. Vuelva a llenar el vaso con, al menos, 120 ml de agua o de zumo de naranja, agite bien y bébaselo inmediatamente. No debe utilizar ningún otro líquido para la dilución, incluyendo zumo de pomelo. La jeringa dosificadora y su tapa deben usarse una sola vez y después desecharse.

Cuando se refrigera, la solución del frasco puede enturbiarse ligeramente. Si ocurre esto, simplemente ponga Rapamune solución oral a temperatura ambiente y agite suavemente. La presencia de esta turbidez no afecta a la calidad de Rapamune.
Si toma más Rapamune del que debe
Si ha tomado más medicamento del que se le dijo, contacte con lo antes posible su médico o acuda a urgencias del hospital más cercano. Lleve siempre con usted el frasco del medicamento con la etiqueta, aunque esté vacío.
Si olvidó tomar Rapamune
Si olvidó tomar Rapamune, tómeselo en cuanto se acuerde pero no dentro de las 4 horas siguientes a la dosis de ciclosporina. Después de esto, continúe tomando el medicamento de la manera habitual. No tome una dosis doble para compensar las dosis olvidadas, y siempre tome Rapamune y ciclosporina con una diferencia de aproximadamente 4 horas. Si olvida por completo tomar una dosis de Rapamune, debe informar a su médico.
Si interrumpe el tratamiento con Rapamune
No deje de tomar Rapamune a menos que su médico le diga que lo haga, ya que se arriesgaría a perder el trasplante.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Deberá acudir a sumédico inmediatamentesi presenta síntomas como hinchazón de cara, lengua y/o fondo de la boca (faringe) y/o dificultad para respirar (angioedema), o descamación de la piel (dermatitis exfoliativa). Podría tratarse de síntomas de una reacción alérgica grave.
Daño renal conbajos recuentos sanguíneos de células (púrpura trombocitopénica/síndrome hemolítico urémico)
Cuando se toma con medicamentos denominados inhibidores de la calcineurina (ciclosporina o tacrolimus) Rapamune puede incrementar el riesgo de un cuadro que combina daño renal con bajos recuentos sanguíneos de plaquetas y glóbulos rojos, con o sin irritación de la piel (púrpura trombocitopénica/síndrome hemolítico urémico). Si experimenta síntomas como moratones, erupciones de la piel, cambios en la orina, cambios de humor o cualquier otro síntoma que considere grave, inusual o prolongado en el tiempo, póngase en contacto con su médico.
Infecciones
Rapamune disminuye los mecanismos de defensa de su cuerpo. Como consecuencia, su cuerpo no será tan bueno como solía luchando contra las infecciones. Por lo tanto, si está tomando Rapamune, puede coger más infecciones de lo habitual, como infecciones de la piel, boca, estómago e intestino, pulmones y tracto urinario (ver listado más abajo). Debe contactar con su médico si experimenta síntomas que considera graves, inusuales o prolongados en el tiempo.
Frecuencia de efectos adversos
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- acumulación de líquidos alrededor del riñón
- hinchazón del cuerpo, incluyendo las manos y los pies
- dolor
- fiebre
- dolor de cabeza
- aumento de la tensión arterial
- dolor de estómago, diarrea, estreñimiento, náuseas
- disminución del número de glóbulos rojos, disminución del número de plaquetas
- aumento de las grasas en sangre (colesterol y/o triglicéridos), aumento del azúcar en sangre, disminución del potasio en sangre, disminución del fósforo en sangre, aumento de la lactato-deshidrogenasa en sangre, aumento de la creatinina en sangre
- dolor de articulaciones
- acné
- infección del tracto urinario
- neumonía y otras infecciones causadas por bacterias, virus y hongos
- disminución de las células de la sangre que luchan contra las infecciones (glóbulos blancos)
- diabetes
- anomalías en las pruebas que miden la función del hígado, elevación de las enzimas del hígado AST y/o ALT
- erupción en la piel
- elevación de las proteínas en orina
- trastornos menstruales (incluyendo períodos ausentes, poco frecuentes o abundantes)
- cicatrización lenta (esto puede incluir la separación de las capas de una herida quirúrgica o línea de sutura)
- aumento de la frecuencia cardiaca
- hay una tendencia general a que los fluidos se acumulen en diversos tejidos
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- infecciones (incluyendo infecciones potencialmente mortales)
- coágulos de sangre en piernas
- coágulos de sangre en los pulmones
- llagas en la boca
- acumulación de líquido en el abdomen
- lesión del riñón con disminución del número de plaquetas y de glóbulos rojos de la sangre, con o sin erupción cutánea (síndrome hemolítico urémico)
- disminución del número de un tipo de glóbulos blancos llamados neutrófilos
- deterioro del hueso
- inflamación que pueden dar lugar a una lesión pulmonar, acumulación de líquido alrededor de los pulmones
- hemorragias nasales
- cáncer de piel
- infección en el riñón
- quistes en los ovarios
- acumulación de fluidos en la membrana que rodea el corazón que, en algunos casos, puede disminuir la capacidad del corazón para bombear sangre
- inflamación del páncreas
- reacciones alérgicas
- herpes
- infección por citomegalovirus
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- cáncer del tejido linfático (linfoma/trastorno linfoproliferativo post-trasplante), disminución conjunta de glóbulos rojos, glóbulos blancos y plaquetas
- hemorragia en los pulmones
- proteínas en orina, en ocasiones grave y asociada a efectos adversos, como hinchazón
- proceso cicatricial del riñón que puede reducir la función del riñón
- exceso de fluidos en los tejidos debido a función linfática irregular
- disminución del número de plaquetas en la sangre, con o sin erupción en la piel (púrpura trombocitopénica)
- reacciones alérgicas graves que pueden provocar la descamación de la piel
- tuberculosis
- infección por virus de Epstein-Barr
- diarrea infecciosa por Clostridium difficile
- lesión grave del hígado
Raros: pueden afectar hasta 1 de cada 1.000 personas
- depósito de proteínas en los saquitos aéreos de los pulmones que puede interferir con la respiración
- reacciones alérgicas graves que pueden afectar a los vasos sanguíneos (ver apartado de reacciones alérgicas)
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles
- Síndrome de encefalopatía posterior reversible (PRES) que es un síndrome grave del sistema nervioso que tiene los siguientes síntomas: dolor de cabeza, nauseas, vómitos, confusión, convulsiones y pérdida de visión. Si padeciera más de uno de estos síntomas, contacte con su médico.
Los pacientes con S-LAM experimentaron efectos adversos similares a los de los pacientes con trasplante renal, con la adición de pérdida de peso, que puede afectar a más de 1 de cada 10 personas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rapamune
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “EXP”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8ºC).
Conserve Rapamune solución oral en su frasco original para protegerlo de la luz.
Una vez abierto el frasco, el contenido debe mantenerse refrigerado y usarse antes de 30 días. Si es necesario, puede conservar el frasco a temperatura ambiente hasta 25ºC por un periodo de tiempo corto, pero no más de 24 horas.
Una vez que la jeringa de dosificación haya sido llenada con Rapamune solución oral, debe ser conservada a temperatura ambiente, pero no por encima de 25ºC, por un periodo máximo de 24 horas.
Una vez que el contenido de la jeringa de dosificación haya sido diluido con agua o con zumo de naranja, la preparación debe ser bebida inmediatamente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Rapamune
El principio activo es sirolimus. Cada ml de Rapamune solución oral contiene 1 mg de sirolimus.
Los demás componentes son:
Polisorbato 80 (E433) y phosal 50 PG (fosfatidilcolina, propilenglicol [E1520], mono y diglicéridos, etanol, ácidos grasos de soja y palmitato de ascorbilo).
Este medicamento contiene aproximadamente 350 mg de propilenglicol (E1520) en cada ml.
Aspecto del producto y contenido del envase
Rapamune solución oral es una solución oral entre amarillo pálido y amarillo que se presenta en frascos de 60 ml.
Cada estuche contiene: 1 frasco (vidrio topacio) que contiene 60 ml de Rapamune solución, un adaptador de jeringa, 30 jeringas dosificadoras (plástico topacio) y una caja para transportar la jeringa.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la Autorización de Comercialización: Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelles Bélgica | Responsable de la fabricación: Pfizer Service Company BV Hoge Wei 10 1930 Zaventem Bélgica |
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/BelgienLuxembourg/Luxemburg Pfizer NV/SA Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. +3705 2514000 |
???????? ??????? ?????????? ????, ???? ???????? Te?: +359 2 970 4333 | Magyarország Pfizer Kft. Tel: +36 1 488 3700 |
Ceská Republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +35621 344610 |
Danmark Pfizer ApS Tlf: +45 44 201 100 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland Pfizer Pharma GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλ?δα PFIZER ΕΛΛΑΣ A.E.Τηλ.: +30 210 6785 800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel:+34914909900 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Slovenija Pfizer Luxembourg SARL, Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: +386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: +1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská Republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf Tel: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
K?προς PFIZER ΕΛΛΑΣ Α.Ε. (Cyprus Branch) T??: +357 22 817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel. +371 67035775 |
Fecha de la última revisión de este prospecto: 01/2025.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RAPAMUNE 1 mg/ml SOLUCION ORALForma farmacéutica: COMPRIMIDO, 0,5 mgPrincipio activo: SirolimusFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: COMPRIMIDO, 1 mgPrincipio activo: SirolimusFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: COMPRIMIDO, 2 mgPrincipio activo: SirolimusFabricante: Pfizer Europe Ma EeigRequiere receta
Médicos online para RAPAMUNE 1 mg/ml SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RAPAMUNE 1 mg/ml SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






