Plegridy 125 microgramos SOLUCION INYECTABLE EN JERINGA PRECARGADA SC

Cómo usar Plegridy 125 microgramos SOLUCION INYECTABLE EN JERINGA PRECARGADA SC
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Plegridy 63 microgramos solución inyectable en jeringa precargada
Plegridy 94 microgramos solución inyectable en jeringa precargada
Plegridy 125 microgramos solución inyectable en jeringa precargada
peginterferón beta-1a
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero,incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Plegridy y para qué se utiliza
- Qué necesita saber antes de empezar a usar Plegridy
- Cómo usar Plegridy
- Posibles efectos adversos
- Conservación de Plegridy
- Contenido del envase e información adicional
- Instrucciones para la inyección de la jeringa precargada de Plegridy
1. Qué es Plegridy y para qué se utiliza
Qué es Plegridy
El principio activo en Plegridy es peginterferón beta-1a. Peginterferón beta-1a es una forma modificada de interferón de acción larga. Los interferones son sustancias naturales producidas en el organismo que ayudan a proteger de las infecciones y enfermedades.
Para qué se utiliza Plegridy
Este medicamento se utiliza para tratar la Esclerosis Múltiple (EM) Remitente Recidivante en adultos de 18 años de edad o mayores.
La EM es una enfermedad de larga duración que afecta al sistema nervioso central (SNC), incluido el cerebro y la médula espinal, en la que el sistema inmunológico del organismo (sus defensas naturales) daña la capa protectora (mielina) que rodea los nervios del cerebro y de la médula espinal. Esto altera los mensajes entre el cerebro y las demás partes del organismo que provoca los síntomas de la EM.
Los pacientes con EM remitente recidivante tienen periodos en los que la enfermedad no está activa (remisión) entre exacerbaciones de los síntomas (brotes).
Cada paciente tiene unos síntomas específicos de EM.Pueden ser:
- sensación de inestabilidad o mareo, problemas para caminar, rigidez y espasmos musculares, cansancio, entumecimiento en la cara, brazos o piernas;
- dolor agudo o crónico, problemas vesicales e intestinales, problemas sexuales y problemas visuales;
- dificultad para pensar y concentrarse, depresión.
¿Cómo actúa Plegridy?
Plegridy parece que actúa impidiendo que el sistema inmunitario dañe el cerebro y la médula espinal.Esto puede ayudar a reducir el número de brotes que tiene y a ralentizar los efectos discapacitantes de la EM. El tratamiento con Plegridy puede ayudar a evitar que empeore, aunque no curará la EM.
2. Qué necesita saber antes de empezar a usar Plegridy
No use Plegridy
- si es alérgicoal peginterferón beta-1a, interferón beta-1a o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Ver los síntomas de una reacción alérgica en la sección 4;
- si padece una depresión graveo pensamientos suicidas.
Advertencias y precauciones
Consulte a su médicosi ha tenido alguna vez:
- depresióno problemas que afecten a su estado de ánimo;
- pensamientos suicidas.
- Su médico puede aún prescribirle Plegridy, pero es importante que sepa si ha tenido depresión u otro problema similar que afecte a su estado de ánimo en el pasado.
Consulte a su médico, farmacéutico o enfermeroantes de inyectar Plegridy si tiene alguna de las afecciones siguientes,ya que pueden empeorar mientras usa Plegridy:
- problemas renales o hepáticos graves;
- irritación en el lugar de inyección,que puede producir daños en la piel y en los tejidos
(necrosis en el lugar de inyección). Cuando esté listo para administrarse, siga cuidadosamente las instrucciones descritas en la sección 7 “Instrucciones para la inyección de la jeringaprecargadadePlegridy”, al final de este prospecto. Así se reduce el riesgo de reacciones en el lugar de inyección;
- epilepsiau otros trastornos convulsivos, no controlados con medicación;
- problemas de corazónque puedan producir síntomas como dolor torácico (angina),especialmente después de cualquier actividad; hinchazón de tobillos, dificultad respiratoria (insuficiencia cardíaca congestiva)o un ritmo cardíaco irregular (arritmias);
- problemas de tiroides;
- recuentos bajos de leucocitos o plaquetas, lo que puede incrementar el riesgo de infección o hemorragia.
Otras cosas a considerar cuando use Plegridy
- Necesitará análisis de sangre para determinar sus números de células sanguíneas, bioquímica sanguínea y sus niveles de enzimas hepáticas. Estas pruebas le serán realizadas antes de empezar a usar Plegridy, a intervalos periódicos tras iniciar el tratamiento con Plegridy y luego periódicamente durante el tratamiento, incluso si no tiene síntomas concretos. Estos análisis de sangre se sumarán a las pruebas que se realizan normalmente para controlar su EM.
- El funcionamiento de su glándula tiroides se revisará regularmente o en cualquier momento que su médico lo considere necesario.
- Pueden formarse coágulos de sangre en los vasos sanguíneos pequeños durante el tratamiento. Estos coágulos de sangre podrían afectar a sus riñones. Esto puede ocurrir tras varias semanas o varios años después de comenzar el tratamiento con Plegridy. Su médico posiblemente quiera realizar controles de su tensión arterial, su sangre (recuento de plaquetas) y su función renal.
Si accidentalmente se pincha o pincha a otra persona con la aguja de Plegridy, se debe lavar con agua y jabón inmediatamentela zona afectada y ponerse en contactolo antes posible con un médico oenfermero.
Niños y adolescentes
Plegridy no debe usarseen niños y adolescentes menores de 18 años. No se conoce la seguridad y eficacia de Plegridy en este grupo de edad.
Otros medicamentos y Plegridy
Plegridy se debe usar con cuidado cuando se administra con otros medicamentos que se procesan en el organismo por un grupo de proteínas llamadas “citocromo P450” (p. ej., algunos medicamentos utilizados para la epilepsia o la depresión).
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, especialmente aquellos utilizados para tratar la epilepsia o la depresión. Esto incluye cualquier medicamento obtenido sin receta médica.
En alguna ocasión será necesario que recuerde a otros profesionales médicos que está siendo tratado con Plegridy, por ejemplo, si le prescriben otros medicamentos o si le realizan un análisis de sangre. Plegridy puede interaccionar con otros medicamentos o con el resultado de la prueba.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se esperan efectos dañinos en el lactante. Plegridy se puede utilizar durante la lactancia.
Conducción y uso de máquinas
La influencia de Plegridy sobre la capacidad para conducir y utilizar máquinas es nula o insignificante. Plegridy contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg); esto es, esencialmente “exento de sodio”.
3. Cómo usar Plegridy
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dosis habitual
Una inyección de Plegridy 125 microgramos cada 14 días (cada dos semanas). Intente usar Plegridy a la misma hora el mismo día cada vez que se inyecte.
Comienzo de Plegridy
Si va a utilizar Plegridy por primera vez, su médico puede aconsejarle que empiece a aumentar gradualmente la dosis para que pueda acostumbrarse a los efectos de Plegridyantes de administrar la dosis completa. Se le suministrará un envase de inicio que contiene las 2 primeras inyecciones: una jeringa naranja con Plegridy 63 microgramos (para el día 0) y una jeringa azul con Plegridy
94 microgramos (para el día 14).
Después se le suministrará un envase de mantenimiento que contiene jeringas grises con Plegridy 125 microgramos (para el día 28 y luego cada dos semanas).
Lea las instrucciones de la sección 7 “ Instrucciones para la inyección de la jeringa precargadadePlegridy” al final de este prospecto antes de empezar a usar Plegridy.
Utilice el recuadro de registro impreso en el interior de la tapa del envase de inicio para llevar un registro de las fechas de las inyecciones.
Autoinyección
Plegridy se inyecta debajo de la piel (inyección subcutánea). Alterne el lugar de inyección. No utilice siempre el mismo lugar de inyección para las demás inyecciones.
Puede inyectarse Plegridy sin la ayuda de su médico si le han enseñado a hacerlo.
- Consulte y siga los consejos dados en las instrucciones de la sección 7 “Instrucciones para lainyección de la jeringa precargada de Plegridy” antes de empezar.
- Si tiene problemaspara manejar la jeringa, hable con su médico o enfermero que le podrán ayudar.
Duración del tratamiento con Plegridy
Su médico le informará durante cuánto tiempo debe utilizar Plegridy. Es importante que utilice Plegridy de forma regular. No haga ningún cambio que no le haya indicado su médico.
Si usa más Plegridy del que debe
Solo debe inyectarse Plegridy una vez cada 2 semanas.
- Si se ha administrado más de una inyección de Plegridy en un plazo de 7 días, póngase encontacto inmediatamente con su médico o enfermero.
Si olvidó usar Plegridy
Debe inyectarse Plegridy una vez cada 2 semanas. Este régimen periódico ayuda a administrar el tratamiento lo más uniformemente posible.
Si se olvida administrarse en su día habitual, inyéctese una dosis tan pronto como sea posible y continúe de la forma habitual. Sin embargo, no se inyecte más de una vez en un periodo de 7 días. No se administre dos inyecciones para compensar la inyección olvidada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
- Problemas hepáticos
(frecuentes: pueden afectar hasta 1 de cada 10 personas)
Si presenta alguno de los síntomas siguientes:
- coloración amarillenta de la piel o de la parte blanca de los ojos;
- picor generalizado;
- sensación de malestar (náuseas y vómitos);
- hematomas que aparecen fácilmente en la piel.
- Póngase en contacto con un médico inmediatamenteya que pueden ser manifestaciones de un posible problema hepático.
- Depresión
(frecuentes: pueden afectar hasta 1 de cada 10 personas)
Si presenta:
- sensación inusual de tristeza, ansiedad o desesperanza; o
- tiene pensamientos suicidas.
- Póngase en contacto con un médico inmediatamente.
- Reacciones alérgicas graves
(poco frecuentes: pueden afectar hasta 1 de cada 100 personas)
Si presenta alguna de las siguientes:
- dificultad para respirar;
- hinchazón alrededor de la cara (labios, lengua o garganta);
- erupción o enrojecimiento de la piel.
- Póngase en contacto con un médico inmediatamente.
- Convulsiones
(poco frecuentes: pueden afectar hasta 1 de cada 100 personas)
Si presenta una crisis convulsiva o un ataque epiléptico.
- Póngase en contacto con un médico inmediatamente.
- Daño en el lugar de inyección
(raros: pueden afectar hasta 1 de cada 1.000 personas)
Si presenta alguno de los síntomas siguientes:
- cualquier herida abierta en la piel junto con hinchazón, inflamación o salida de líquido alrededor del lugar de inyección.
- Póngase en contacto con un médico para que le aconseje.
- Problemas renales que incluyen cicatrización que puede reducir su función renal
(raros: pueden afectar hasta 1 de cada 1.000 personas)
Si presenta alguno o todos estos síntomas:
- orina con espuma;
- fatiga;
- hinchazón, especialmente de tobillos y párpados, y aumento de peso.
- Póngase en contacto con un médico ya que pueden ser manifestaciones de un posible problema renal.
- Problemas de la sangre
(raros: pueden afectar hasta 1 de cada 1.000 personas)
Pueden ocurrir los siguientes: coágulos de sangre en vasos sanguíneos pequeños que pueden afectar a sus riñones (púrpura trombocitopénica trombótica o síndrome urémico hemolítico). Los síntomas pueden incluir un aumento de moretones, sangrado, fiebre, debilidad extrema, dolor de cabeza, mareo o aturdimiento. Su médico puede encontrar alteraciones en su sangre y en su función renal.
Si presenta alguno o todos estos síntomas:
- aumento de hematomas o sangrado;
- debilidad extrema;
- dolor de cabeza, mareos o aturdimiento.
- Póngase en contacto con un médico inmediatamente.
Otros efectos adversos
Efectos adversos muy frecuentes
(pueden afectar a más de 1 de cada 10 personas)
- síntomas seudogripales. Estos síntomas realmente no corresponden a la gripe, ver a continuación. No los puede transmitir a nadie;
- dolor de cabeza;
- dolores musculares (mialgia);
- dolor en las articulaciones, brazos, piernas o cuello (artralgia);
- escalofríos;
- fiebre;
- debilidad y cansancio (astenia);
- enrojecimiento, picor o dolor alrededor del lugar de inyección.
- Si le preocupa alguno de estos efectos, póngase en contacto con un médico.
Síntomas seudogripales
Los síntomas seudogripales son más frecuentes cuando utiliza Plegridy por primera vez. A medida que sigue usando las inyecciones, los síntomas van desapareciendo gradualmente. Ver a continuación algunas formas sencillas de combatir estos síntomas seudogripales si los sufre.
Tres sencillas formas de ayudar a reducir el impacto de los síntomas seudogripales:
- Tenga en cuenta la hora de administración de la inyección de Plegridy. El comienzo y la finalización de los síntomas seudogripales son diferentes para cada paciente. Por término medio los síntomas seudogripales comienzan, de forma aproximada, 10 horas después de la inyección y duran entre 12 y 24 horas.
- Tome paracetamol o ibuprofeno media hora antes de la inyección de Plegridy y continúe tomándolo durante el tiempo que duren los síntomas seudogripales. Pregunte a su médico o farmacéutico qué cantidad tomar y durante cuánto tiempo.
- Si tiene fiebre, beba bastante agua para mantenerse hidratado.
Efectos adversos frecuentes
(pueden afectar hasta 1 de cada 10 personas)
- sensación de malestar (náuseas o vómitos);
- pérdida de pelo (alopecia);
- picor de piel (prurito);
- aumento de la temperatura corporal;
- cambios alrededor del lugar de la inyección como hinchazón, inflamación, hematoma, calor, exantema o cambio de color;
- cambios en la sangre pueden producir cansancio o capacidad reducida para luchar contra las infecciones;
- aumento de enzimas hepáticas en la sangre (aparecerán en los análisis de sangre).
- Si le preocupa alguno de estos efectos, póngase en contacto con un médico.
Efectos adversos poco frecuentes
(pueden afectar hasta 1 de cada 100 personas)
- urticaria;
- cambios en la sangre que pueden ser la causa de los hematomas o sangrado sin explicación.
- Si le preocupa alguno de estos efectos, póngase en contacto con un médico.
Frecuencia no conocida
(no puede estimarse a partir de los datos disponibles)
- Hipertensión arterial pulmonar: Enfermedad en la que se produce un gran estrechamiento de los vasos sanguíneos de los pulmones que provoca un aumento de la presión en los vasos sanguíneos que transportan la sangre del corazón a los pulmones. La hipertensión arterial pulmonar se observó en distintos momentos, incluso varios años después del inicio del tratamiento con medicamentos que contienen interferón beta.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
Con objeto de mejorar la trazabilidad de este medicamento, su médico o farmacéutico debe registrar el nombre y el número de lote del medicamento que se le ha administrado en su historia clínica. También puede que usted desee tomar nota de esta información por si se le pide en el futuro.
5. Conservación de Plegridy
Mantener este medicamento fuera de la vista y del alcance de los niños.
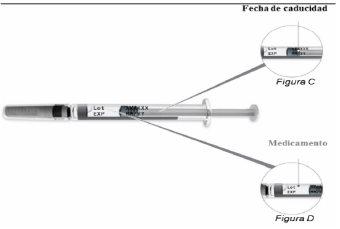
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y la caja después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
- Conservar en el embalaje original para proteger de la luz. Únicamente abrir el envase cuando se necesite una nueva jeringa.
- Conservar en nevera(frigorífico), entre 2ºC y 8ºC.
- No congelar. Deseche cualquier Plegridy que se haya congelado por accidente.
- Plegridy puede conservarse fuera de la nevera a temperatura ambiente (hasta 25°C) durante un tiempo máximo de 30 días pero debe conservarse protegido de la luz.
- Los envases pueden sacarse y volverse a meter en la nevera en más de una ocasión si es necesario.
- Asegúrese de que el tiempo que pasan las jeringas fuera de la nevera no es mayor de30 días en total.
- Deseche cualquier jeringa que haya estado fuera de la nevera durante más de 30 días.
- Si no se sabe con certeza el número de días que ha tenido una jeringa fuera de la nevera, deseche la jeringa.
- No utilice este medicamento si observa que:
- la jeringa está rota.
- la solución presenta un cambio de color, está turbia o puede ver partículas en suspensión.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Plegridy
El principio activo es peginterferón beta-1a.
Cada jeringa precargada de 63 microgramos contiene 63 microgramos de peginterferón beta-1a en 0,5 ml de solución inyectable.
Cada jeringa precargada de 94 microgramos contiene 94 microgramos de peginterferón beta-1a en 0,5 ml de solución inyectable.
Cada jeringa precargada de 125 microgramos contiene 125 microgramos de peginterferón beta-1a en 0,5 ml de solución inyectable.
Los demás componentes son acetato de sodio trihidrato, ácido acético glacial, hidrocloruro de arginina, polisorbato 20 y agua para preparaciones inyectables (ver sección 2 “Plegridy contiene sodio”).
Aspecto del producto y contenido del envase
Plegridy es una solución inyectable transparente e incolora en una jeringa de vidrio precargada con una aguja acoplada.
Tamaños de envase:
- El envase de inicio de Plegridy contiene una jeringa precargada naranja de 63 microgramos y una jeringa precargada azul de 94 microgramos.
- Las jeringas grises de 125 microgramos se suministran en un envase que contiene 2 o 6 jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Responsable de la fabricación
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1
DK-3400 Hillerød
Dinamarca
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
Fecha de la última revisión de este prospecto:01/2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- Instrucciones para la inyección de la jeringa precargada de Plegridy Cómo inyectar Plegridy
Lea las instrucciones antes de empezar a usar Plegridy y cada vez que obtenga una nueva receta. Puede haber información nueva. Esta información no sustituye la conversación con su médico o enfermero sobre su enfermedad o su tratamiento.
Nota:
- Antes de usar la jeringa precargada de Plegridy por primera vez, su médico o enfermero debe enseñarle a usted o a su cuidador cómo preparar e inyectar la jeringa precargada de Plegridy.
- La jeringa precargada de Plegridy sirve para inyectar el medicamento por debajo de la piel únicamente (vía subcutánea).
- Cada jeringa precargada de Plegridy se puede usar solo una vez.
Nocomparta la jeringa precargada de Plegridy con ninguna otra persona para evitar el contagio de infecciones.
Nouse más de una jeringa precargada cada 14 días (cada 2 semanas).
Nouse la jeringa si se ha caído o presenta daños visibles.
Calendario de administración
El envase de inicio contiene sus dos primeras inyecciones para ajustar de forma gradual su dosis. Elija la jeringa correcta del envase.
Cuándo | Qué dosis | Qué envase |
Día 0 (63 microgramos) | Primera inyección: 63 microgramos elija la jeringa naranja |
|
Día 14 (94 microgramos) | Segunda inyección: 94 microgramos elija la jeringa azul | |
Día 28 y cada dos semanas a partir de entonces (125 microgramos) | Inyección de dosis completa: 125 microgramos elija la jeringa gris |
|
No usemás de una jeringa precargada en un periodo de 14 días (cada 2 semanas).
Materiales necesarios para la inyección de Plegridy
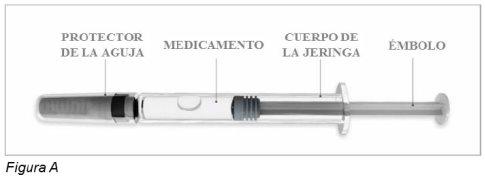
Jeringa precargada de Plegridy (ver Figura A)
Antes de usar – Partes de la jeringa precargada de Plegridy (Figura A)
|
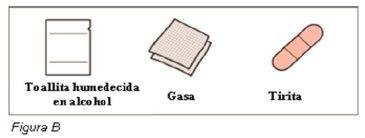
Materiales adicionales no incluidos en el envase (ver Figura B):
- toallita humedecida en alcohol
- gasa
- tirita
Pregunte a su médico, farmacéutico o enfermero para obtener instrucciones sobre cómo desechar las jeringas utilizadas.
|
Preparación para la inyección
Paso 1: Saque la jeringa precargada de la nevera
- Saque un envase de Plegridy de la nevera y elija la jeringa precargada apropiada del envase.
- Cierre el envase y guárdelo de nuevo en la nevera después de coger la jeringa precargada.
- Deje que la jeringa precargada de Plegridy alcance la temperatura ambiente durante al menos 30 minutos.
Noutilice fuentes externas de calor, como agua caliente, para calentar la jeringa precargada dePlegridy.
Paso 2: Reúna los materiales y lávese las manos
- Para la preparación, utilice una superficie plana, limpia y bien iluminada, por ejemplo una mesa. Reúna todos los materiales necesarios para administrarse o para que le administren la inyección.
- Lávese las manos con agua y jabón.
Paso 3: Compruebe la jeringa precargada de Plegridy | |
No use la jeringa precargada dePlegridy una vez superada la fecha de caducidad.
No use la jeringa precargada dePlegridy si el líquido tiene color, está turbio o contiene partículas en suspensión. o Puede que vea burbujas de aire en el medicamento de Plegridy. Esto es normal y no es necesario eliminar las burbujas antes de la inyección. |
|
Administración de la inyección
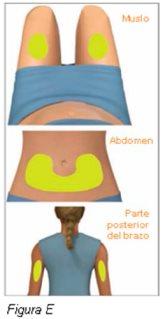
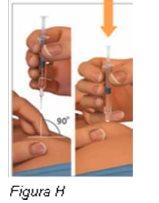
Paso 4: Elija y limpie el lugar de inyección | |
Noinyecte directamente en el ombligo. Noinyecte en una zona del cuerpo que tenga la piel irritada, dolorida, enrojecida, con hematoma, tatuada, infectada o cicatrizada.
Notoque ni sople esta zona antes de administrar la inyección. |
|
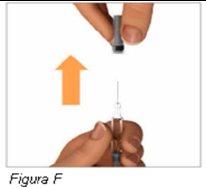
Paso 5: Retire con firmeza el protector de la aguja | |
Tenga precauciónal retirar el protector de la aguja para evitar pincharse con la aguja. Notoque la aguja. Advertencia: novuelva a poner el protector de la aguja en la jeringa precargada de Plegridy. Podría pincharse con la aguja. |
|
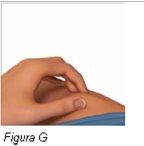
Paso 6: Pellizque suavemente el lugar de inyección | |
|
|
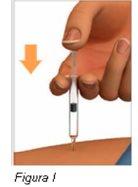
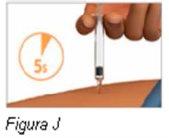
Paso 7: Inyecte el medicamento | |
Notire del émbolo hacia atrás. |
|
Nosaque la jeringa precargada de Plegridy del lugar de inyección hasta que haya entrado el émbolo hasta el fondo. |
|
|
|
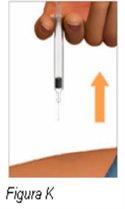
Paso 8: Saque la jeringa precargada del lugar de inyección | |
Advertencia: novuelva a poner el protector de la aguja en la jeringa precargada de Plegridy. Podría pincharse con la aguja. Novuelva a usar la jeringa precargada dePlegridy. |
|
Después de la inyección
Paso 9: Eliminación de la jeringa precargada de Plegridy utilizada
- Consulte a su médico, farmacéutico o enfermero sobre la forma correcta de eliminar la jeringa utilizada.
Paso 10: Cuidados del lugar de inyección
- En caso necesario, póngase la gasa o la tirita en el lugar de inyección.
Paso 11: Compruebe el lugar de inyección
- Tras 2 horas, compruebe el lugar de inyección por si presenta enrojecimiento, hinchazón o dolor a la palpación.
- Si presenta una reacción cutánea y no desaparece en unos días, póngase en contacto con su médico o enfermero.
Registre la fecha y el lugar
- Registre la fecha y el lugar de administración de cada inyección.
- Para las primeras inyecciones puede utilizar el recuadro de registro impreso en el interior de la tapa del envase de inicio.
Advertencias generales
Noreutilicela jeringa precargada dePlegridy.
Nocomparta la jeringa precargada de Plegridy.
- Mantener la jeringa precargada de Plegridy y todos los medicamentos fuera del alcance de los niños.
Conservación
- Se recomienda conservar en nevera a una temperatura controlada entre 2ºC y 8ºC en el embalaje original cerrado para protegerlo de la luz.
- En caso necesario, se puede conservar Plegridy en el embalaje original cerrado fuera de la nevera a una temperatura de hasta 25ºC durante un periodo de hasta 30 días.
- En caso necesario, se puede sacar Plegridy de la nevera y volverlo a meter. El tiempo total combinado fuera de la nevera a una temperatura de hasta 25ºC no debe superar los
30 días.
Nocongelar ni exponer a temperaturas altas.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a Plegridy 125 microgramos SOLUCION INYECTABLE EN JERINGA PRECARGADA SCForma farmacéutica: INYECTABLE, 125 µgPrincipio activo: Peginterferon beta-1aFabricante: Biogen Netherlands B.V.Requiere recetaForma farmacéutica: INYECTABLE, 125 mcgPrincipio activo: Peginterferon beta-1aFabricante: Biogen Netherlands B.V.Requiere recetaForma farmacéutica: INYECTABLE, 63mcg/94mcgPrincipio activo: Peginterferon beta-1aFabricante: Biogen Netherlands B.V.Requiere receta
Médicos online para Plegridy 125 microgramos SOLUCION INYECTABLE EN JERINGA PRECARGADA SC
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de Plegridy 125 microgramos SOLUCION INYECTABLE EN JERINGA PRECARGADA SC, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes