
PETIDINA BASI 50 MG/ML SOLUCION INYECTABLE EFG

Cómo usar PETIDINA BASI 50 MG/ML SOLUCION INYECTABLE EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Petidina Basi y para qué se utiliza
- Qué necessita saber antes de empezar a usar Petidina Basi
- Consulte a su médico, farmacéutico o enfermero antes de empezar a usar petidina :
- Cómo usar Petidina Basi
- Posibles efectos adversos
- Conservación de Petidina Basi
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Petidina Basi 50 mg/ml solución inyectable EFG
hidrocloruro de petidina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Petidina Basi y para qué se utiliza
2.ué necesita saber antes de empezar a usarp asi
- Cómo usar Petidina Basi
4.osibles efectos adversos
- Conservación de Petidina Basi
6.ontenido del envase e información adicional
1. Qué es Petidina Basi y para qué se utiliza
Petidina pertenece a un grupo de medicamentos llamados analgésicos opioides que se utilizan para aliviar el dolor intenso.
Petidina puede utilizarse en:
- El tratamiento del dolor intenso, incluido el dolor desencadenado por operaciones o fracturas, entre otros;
- El tratamiento del dolor en el parto;
- Como medicación antes de la anestesia.
2. Qué necessita saber antes de empezar a usar Petidina Basi
No use petidina basi
- Si es alérgico a la petidina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene problemas respiratorios graves.
- Si está siendo tratado o ha sido tratado desde hace menos de dos semanas con algún medicamento para la depresión que pertenece al grupo de inhibidores de la monoaminooxidasa (IMAOs), tales como iproniacida, nialamida, fenelzina, moclobemida, toloxatona o selegilina.
- Si está tomando algún medicamento que pertenece al grupo de agonistas-antagonistas opioides, como buprenorfina, nalbufina o pentazocina.
- Si está tomando ritonavir, un medicamento usado en el tratamiento del SIDA.
- Si tiene problemas graves de riñón.
- Si tiene problemas graves de hígado.
- Si se le ha diagnosticado feocromocitoma, un problema de las glándulas suprarrenales.
- Si tiene aumento de la presión del cerebro o si acaba de tener alguna lesión cerebral.
- Si el paciente está en coma.
- Si ha bebido mucho alcohol.
- Si tiene riesgo de obstrucción del intestino.
- Si sufre diarrea intensa causada por antibióticos o intoxicación.
- Si tiene riesgo de presentar convulsiones.
- Si el paciente es un niño de menos de 6 meses.
- Si está en periodo de lactancia.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar petidina :
- En caso de embarazo o si está intentando quedarse embarazada.
- Si tiene asma. Si su asma está controlada puede tomar este medicamento pero con especial cuidado. No debe tomar este medicamento durante un ataque agudo de asma.
- Si tiene bronquitis, acumulación de aire en los pulmones (enfisema), cor pulmonale (un tipo de problema del corazón), obesidad severa o deformidad severa de la columna vertebral.
- Si tiene alguna enfermedad en hígado o vesícula biliar.
- Si tiene problemas de riñón (no se le debe administrar este medicamento si tiene insuficiencia renal severa).
- Si tiene problemas de próstata o dificultad al orinar.
- Si tiene baja actividad del tiroides o de las glándulas suprarrenales.
- Si tiene presión arterial baja, si está en un estado severo de shock, o si se encuentra muy debilitado.
- Se es anciano.
- Si tiene alguna enfermedad del intestino tal como enfermedad de Crohn o colitis ulcerosa.
- Si tiene molestias abdominales recientes cuya causa no ha sido identificada por su médico.
- Si tiene antecedentes de epilepsia (ataques epilépticos).
- Si tiene tendencia al abuso de drogas o ha abusado de drogas en el pasado.
- Si tiene un latido del corazón rápido e irregular.
- Si tiene cáncer o un tipo de anemia denominada anemia de células falciformes.
- Si se administra a niños.
Uso en deportistas
Este medicamento contiene petidina que puede producir un resultado positivo en las pruebas de control de dopaje (ver referencias en la sección 4.4 de ficha técnica).
Otros medicamentos y petidina basi
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Este medicamento no debe utilizarse:
- Si está siendo o ha sido tratado desde hace menos de dos semanas con IMAOs, tales como iproniazida, nialamida, fenelzina, moclobemida, toloxatona o selegilina.
- Si está siendo tratado con medicamentos como buprenorfina, nalbufina o pentazocina.
- Si está siendo tratado con ritonavir.
Existe riesgo de interacción con:
- Alcohol, ya que puede potenciar los efectos sedantes y de disminución de la tensión.
- Antiarrítmicos, como mexiletina.
- Antibacterianos, como ciprofloxacino.
- Antipsicóticos, como clorpromacina.
- Antidiarreicos, como loperamida y caolín, pues existe riesgo de estreñimiento severo.
- Antiepilépticos, como fenobarbital y fenitoina, pues se puede aumentar el efecto depresor.
- Antimuscarínicos como atropina, pues pueden aparecer estreñimiento y retención urinaria severos.
- Metoclopramida y domperidona.
- Cimetidina.
El uso concomitante de hidrocloruro de petidina y de medicamentos sedantes como las benzodiacepinas o medicamentos relacionados aumenta el riesgo de somnolencia, dificultades para respirar (depresión respiratoria), coma y puede ser mortal. Por ello, el uso concomitante sólo debe considerarse cuando no sean posibles otras opciones de tratamiento.
Sin embargo, si su médico le prescribe Petidina Basi junto con medicamentos sedantes, la dosis y duración del tratamiento concomitante debe ser limitada por su médico.
Informe a su médico sobre todos los medicamentos sedantes que esté tomando y siga atentamente las recomendaciones de su médico en cuanto a la dosis. Puede ser útil informar a amigos o familiares para que reconozcan los signos y síntomas mencionados anteriormente. Si tiene estos síntomas, póngase en contacto con su médico.
Uso de petidina basi con alimentos y bebidas
No ingerir bebidas alcohólicas.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Se desconoce si Petidina Basi produce alteraciones en el desarrollo del feto durante el embarazo. Se desaconseja la utilización de este medicamento durante el primer trimestre de embarazo y como medida de precaución, es preferible no utilizar este medicamento durante el segundo y tercer trimestre de embarazo.
Petidina Basi puede causar alteraciones respiratorias y problemas de succión en el recién nacido.
Los niños hijos de madres que reciben tratamiento con petidina durante periodos de tiempo prolongados y desarrollan dependencia, pueden a su vez desarrollar también dependencia y manifestar síntomas de abstinencia después del parto.
Durante el parto aumenta el riesgo para la madre de neumonía.
No se debe utilizar este medicamento durante el periodo de lactancia.
Conducción y uso de máquinas
Pregunte a su médico si puede conducir o utilizar máquinas durante el tratamiento con Petidina Basi. Es importante que antes de conducir o utilizar máquinas, observe como le afecta este medicamento. No conduzca ni utilice máquinas si siente sueño, mareo, tiene visión borrosa o ve doble, o tiene dificultad para concentrarse.
3. Cómo usar Petidina Basi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Si estima que la acción de petidina es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Siga estas instrucciones a menos que su médico le haya dado otras distintas.
Duración del tratamiento
Su médico le indicará la duración de su tratamiento con petidina.
Forma de administración
Petidina Basi puede ser administrado por vía subcutánea (inyección por debajo de la piel), intramuscular (inyección en un músculo) o intravenosa (inyección lenta en una vena), diluyendo el contenido de la ampolla en una solución compatible.
Posología
La dosis de hidrocloruro de petidina deberá ajustarse en función de la intensidad del dolor y la respuesta de cada paciente.
Tratamiento del dolor severo, incluido el dolor tras cirugía
Adultos
- 25 mg – 100 mg cada 4 horas, mediante inyección intramuscular o subcutánea.
- 25 mg – 50 mg cada 4 horas, mediante inyección intravenosa lenta.
Personas de edad avanzada
Los ancianos pueden ser más sensibles a los efectos de hidrocloruro de petidina, especialmente a sus efectos sobre el sistema nervioso central. La dosis inicial no debe exceder los 25 mg, pudiendo ser necesario reducir la dosis total diaria en caso de administraciones repetidas.
Tratamiento del dolor en el parto
50 mg - 100 mg mediante inyección intramuscular o subcutánea, tan pronto como aparezcan contracciones a intervalos regulares. La dosis se puede repetir transcurridas de 1-3 horas si fuera necesario, hasta un máximo de 400 mg en 24 horas.
Medicación preanestésica
Debe administrarse este medicamento aproximadamente 1 hora antes de la intervención.
Adultos
- 50 mg - 100 mg por inyección intramuscular
Ancianos
- 50 mg - 100 mg por inyección intramuscular
Los ancianos pueden ser más sensibles a los efectos de petidina.
Niños
- 1,0 mg/kg - 2,0 mg/kg cada 4 horas, mediante inyección intramuscular.
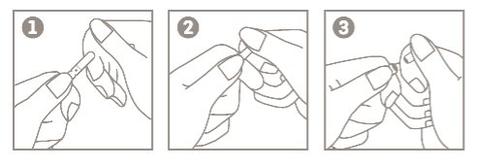
Instrucciones para la apertura de las ampollas OPC (One-Point-Cut):
- Sostenga el cuerpo de la ampolla entre el pulgar y el dedo índice, con el punto hacia arriba;
- Coloque el dedo índice de la otra mano sosteniendo la parte superior de la ampolla. Coloque el pulgar sobre el punto;
- Con los dedos índices cerca uno del otro, presione el área del punto para abrir la ampolla.
Uso en niños
Este medicamento solo está indicado para uso como medicación previa a la anestesia. Está contraindicado en niños menores de 6 meses.
Uso en personas de edad avanzada (> 65 años)
Los mayores de 65 años pueden ser más sensibles a la petidina, por lo que su médico puede prescribirle una dosis más baja.
Poblaciones especiales
Pacientes con problemas de hígado
Debe reducirse la dosis en caso de problemas leves o moderados que afecten al hígado. Está contraindicado en caso de insuficiencia hepática grave.
Pacientes con problemas de riñón
Debe reducirse la dosis en caso de problemas leves o moderados que afecten al riñón. Su uso está contraindicado en caso de insuficiencia renal grave.
Si usa más petidina basi del que debe
Si usted ha utilizado más Petidina Basi de lo que debe, consulte inmediatamente a su médico o farmacéutico, indicando el medicamento y la cantidad utilizada. Es conveniente que lleve el envase y el prospecto del medicamento a su médico o farmacéutico.
La administración de una dosis alta de petidina puede producir disminución de la capacidad para respirar, coma, estupor, y disminución del tamaño de las pupilas. Si la sobredosis es muy alta se puede llegar a producir una parada de la respiración y muerte.
Los efectos excitantes de petidinaincluyen temblores, tics musculares y convulsiones. Otros síntomas que pueden aparecer con dosis altas incluyen frío, piel fría y húmeda y disminución de la temperatura del cuerpo, debilidad muscular, bajada de la tensión, disminución de los latidos del corazón, reducción de la circulación de la sangre, parada del corazón, confusión, fuerte mareo, mucho sueño, nerviosismo o mucha inquietud, alucinaciones, hinchazón de los pulmones y problemas en el riñón.
Si olvidó usar petidina basi
En caso de olvido de una dosis, utilice el medicamento lo antes posible, continuando el tratamiento de la forma prescrita. Sin embargo, cuando esté próxima la siguiente dosis, es mejor que no le administren la dosis olvidada y esperar a la siguiente. No solicite la administración de una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con petidina basi
Su médico le indicará la duración de su tratamiento con Petidina Basi. No suspenda el tratamiento antes, ya que podrían volver a aparecer los dolores y podría sufrir síntomas de abstinencia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos han sido clasificados en frecuencias según lo siguiente: Muy frecuentes (pueden afectar a más de 1 de cada 10 personas); frecuentes (pueden afectar entre 1 y 10 de cada 100 personas); poco frecuentes (pueden afectar entre 1 y 10 de cada 1.000 personas); raros (pueden afectar entre 1 y 10 de cada 10.000 personas); muy raros (pueden afectar a menos de 1 por cada 10.000 personas), frecuencia no conocida (no puede estimarse a partir de los datos disponibles).
Trastornos cardíacos
Frecuentes: disminución de la tensión, disminución o aumento del ritmo del corazón, palpitaciones.
Trastornos del sistema nervioso
Frecuentes: adormecimiento, mareos, sudores, confusión, euforia o sensación aumentada de bienestar, alucinaciones, dolor de cabeza, convulsiones o temblor, disminución del ritmo de la respiración.
Trastornos oculares
Frecuentes: disminución del tamaño de las pupilas y otras alteraciones de la visión.
Trastornos gastrointestinales
Frecuentes: náuseas y vómitos, estreñimiento, boca seca.
Trastornos renales y urinarios
Poco frecuentes: retención de orina y falta de orina.
Trastornos de la piel y del tejido subcutáneo
Raros: reacciones alérgicas, reacciones en el lugar de la inyección.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto consulte a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Petidina Basi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25 °C.
No utilice este medicamento si observa la presencia de partículas visibles.
Una vez abiertas las ampollas o diluido su contenido, se debe administrar inmediatamente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Petidina Basi
- El principio activo es hidrocloruro de petidina.
Cada ml de solución inyectable contiene 50 mg de hidrocloruro de petidina.
- Los demás componentes son ácido clorhídrico, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Petidina Basi se presenta en forma de solución inyectable límpida e incolora o casi incolora, en ampolla de vidrio incoloro de tipo I de 1 o 2 ml.
Tamaño del envase: 10 ampollas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Laboratórios Basi – Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lote 15
3450-232 Mortágua
Portugal
Tel: + 351 231 920 250
Fax: + 351 231 921 055
E-mail: [email protected]
Responsable de la fabricación
Laboratórios Basi – Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lotes 8, 15, 16
3450-232 Mortágua
Portugal
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Representante Local
Laphysan, S.A.U.
Calle Anabel Segura 11,
Complejo Empresarial Albatros, Edificio A, Planta 4, puerta D,
28108 Alcobendas (Madrid)
Fecha de la última revisión de este prospecto:
Febrero 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PETIDINA BASI 50 MG/ML SOLUCION INYECTABLE EFGForma farmacéutica: INYECTABLE, 100 mg/2 ml hidrocloruro de petidinaPrincipio activo: pethidineFabricante: Kern Pharma S.L.Requiere recetaForma farmacéutica: INYECTABLE, 50 mg/mlPrincipio activo: pethidineFabricante: Altan Pharmaceuticals SaRequiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 12 MCG/HPrincipio activo: fentaniloFabricante: Aristo Pharma Iberia S.L.Requiere receta
Médicos online para PETIDINA BASI 50 MG/ML SOLUCION INYECTABLE EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PETIDINA BASI 50 MG/ML SOLUCION INYECTABLE EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















