
ORENCIA 125 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar ORENCIA 125 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ORENCIA50mg solución inyectable en jeringa precargada
ORENCIA87,5mg solución inyectable en jeringa precargada
ORENCIA125mg solución inyectable en jeringa precargada
abatacept
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es ORENCIA y para qué se utiliza
- Qué necesita saber antes de empezar a usar ORENCIA
- Cómo usar ORENCIA
- Posibles efectos adversos
- Conservación de ORENCIA
- Contenido del envase e información adicional
1. Qué es ORENCIA y para qué se utiliza
ORENCIA contiene el principio activo abatacept, una proteína que se obtiene en cultivos celulares. ORENCIA disminuye el ataque del sistema inmunológico sobre tejidos normales interfiriendo con las células inmunes (los llamados linfocitos T) que contribuyen al desarrollo de la artritis reumatoide. ORENCIA modula selectivamente la activación de los linfocitos T que participan en la respuesta inflamatoria del sistema inmunitario.
ORENCIA se utiliza para tratar la artritis reumatoide y artritis psoriásica en adultos y también la artritis idiopática juvenil poliarticular en niños a partir de 2 años.
Artritis reumatoide
La artritis reumatoide es una enfermedad sistémica progresiva de larga duración que, si no se trata, puede tener graves consecuencias, como la destrucción de las articulaciones, aumento de la discapacidad e incapacidad para realizar las actividades diarias. En las personas que padecen artritis reumatoide el propio sistema inmunológico del cuerpo ataca a los tejidos normales produciendo dolor e hinchazón en las articulaciones. Esto puede dañar las articulaciones. La artritis reumatoide (AR) afecta a cada persona de forma diferente. En la mayoría de las personas, los síntomas articulares se desarrollan gradualmente a lo largo de varios años. Sin embargo, en algunos pacientes, la AR puede progresar rápidamente e incluso otras personas pueden tener AR durante un período limitado de tiempo y luego entrar en un período de remisión. La AR es habitualmente una enfermedad crónica (a largo plazo), progresiva. Esto significa que, aunque reciba tratamiento, siga teniendo o no síntomas, la AR podría seguir dañando sus articulaciones. Con el mejor plan de tratamiento para usted, podría ser capaz de retrasar este proceso de la enfermedad, lo que podría ayudar a reducir el daño articular a largo plazo, así como el dolor y el cansancio y mejorar su calidad de vida global.
ORENCIA se utiliza para tratar la artritis reumatoide activa de moderada a grave cuando usted no responde suficientemente al tratamiento con otros medicamentos modificadores de la enfermedad o con otro grupo de medicamentos llamados "inhibidores del factor de necrosis tumoral (TNF)". Se utiliza en combinación con un medicamento llamado metotrexato.
ORENCIA también puede ser utilizado con metotrexato para tratar la artritis reumatoide progresiva y con alta actividad sin tratamiento previo con metotrexato.
ORENCIA se utiliza para:
- retrasar el daño de sus articulaciones
- mejorar su función física
Artritis psoriásica
La artritis psoriásica es una enfermedad inflamatoria de las articulaciones, normalmente acompañada de psoriasis, una enfermedad inflamatoria de la piel. Si usted tiene artritis psoriásica activa primero le darán otros medicamentos. Si no responde suficientemente bien a estos medicamentos, puede que le administren ORENCIA para:
- Reducir los signos y síntomas de su enfermedad.
- Reducir el daño en sus huesos y articulaciones.
- Mejorar su función física y su habilidad para realizar las actividades diarias normales.
ORENCIA solo o en combinación con metotrexato se utiliza para tratar la artritis psoriásica.
Artritis idiopática juvenil poliarticular
La artritis idiopática juvenil poliarticular es una enfermedad inflamatoria de larga duración que afecta a una o más articulaciones en niños y adolescentes.
ORENCIA solución inyectable en jeringa precargada se usa en niños y adolescentes con edades de 2 a 17 años cuando un tratamiento previo con un medicamento modificador de la enfermedad no ha funcionado bien o no sea apropiado para ellos. ORENCIA se utiliza habitualmente en combinación con metotrexato, aunque ORENCIA también se puede utilizar solo en caso de que el tratamiento con metotrexato sea inadecuado.
ORENCIA se utiliza para:
- retrasar el daño en las articulaciones
- mejorar la función física
- mejorar otros signos y síntomas de la artritis idiopática juvenil poliarticular
2. Qué necesita saber antes de empezar a usar ORENCIA
No use ORENCIA
- si es alérgicoa abatacept o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una infección grave o no controlada, no comience el tratamiento con ORENCIA. El hecho de tener una infección podría ponerle en riesgo de sufrir efectos graves a causa de ORENCIA.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero:
- si usted presenta reacciones alérgicascomo opresión en el pecho, asma, vértigo grave o mareo, hinchazón o erupción de la piel informe a su médico inmediatamente.
- si usted, su pareja o su cuidador notan una nueva aparición o un empeoramiento de los síntomas neurológicos, incluyendo debilidad muscular general, alteraciones de la visión, dificultad para hablar, un cambio en la forma de caminaro problemas con el equilibrio, cambios en el pensamiento, la memoria y la orientaciónque conducen a confusión y cambios de personalidad, póngase en contacto inmediatamente con su médicoporque pueden ser síntomas de una infección cerebral muy rara, grave y potencialmente mortal llamada leucoencefalopatía multifocal progresiva (LMP).
- si usted presenta cualquier tipo de infección, incluyendo infección prolongada o localizada, o si usted a menudo padece infecciones o si tiene síntomas de presentar una infección (por ejemplo fiebre, malestar, problemas dentales), es importante que informe a su médico. ORENCIA puede disminuir la capacidad de su cuerpo para combatir una infección y el tratamiento puede hacerle más proclive a adquirir infecciones o empeorar cualquier infección que tenga.
- si usted ha padecido tuberculosis (TB)o padece los síntomas de la tuberculosis (tos persistente, pérdida de peso, apatía, fiebre leve) informe a su médico. Antes de utilizar ORENCIA, su médico le hará la prueba de la tuberculosis o una prueba cutánea.
- si usted tiene una hepatitis víricainforme a su médico. Antes de utilizar ORENCIA, su médico puede hacerle una prueba para la hepatitis.
- si usted tiene cáncer, su médico decidirá si puede administrarle ORENCIA.
- si se ha vacunado recientementeo está pensando en vacunarse, informe a su médico. Algunas vacunas no se deben administrar mientras esté en tratamiento con ORENCIA. Consulte con su médico antes de que le administren cualquier vacuna.Ciertas vacunas pueden producir infecciones. Si le administraran ORENCIA durante su embarazo, su bebé podría tener un riesgo más alto de contraer dicha infección durante aproximadamente las 14 semanas después de la última dosis que usted recibió durante su embarazo. Es importante que usted informe a los médicos sobre su bebé y a otros profesionales sanitarios sobre el uso de ORENCIA durante su embarazo para que ellos puedan decidir cuándo se le debe administrar a su bebé cualquier vacuna.
Su médico puede también hacerle pruebas para examinar los valores sanguíneos.
Niños y adolescentes
ORENCIA solución inyectable en jeringa precargada no ha sido estudiado en niños y adolescentes menores de 2 años. Por lo tanto, no se recomienda el uso de ORENCIA solución inyectable en jeringa precargada en esta población de pacientes.
Uso de ORENCIA con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
ORENCIA no se debe utilizarcon medicamentos biológicos para la artritis reumatoide incluyendo inhibidores del TNF como adalimumab, etanercept, e infliximab; no hay suficiente evidencia para recomendar que se administre con anakinra y rituximab.
ORENCIA se puede utilizarcon otros medicamentos habitualmente utilizados para tratar la artritis reumatoide, como esteroides o analgésicos incluyendo antiinflamatorios no esteroideos como ibuprofeno o diclofenaco.
Pida consejo a su médico o farmacéutico antes de tomar cualquier otro medicamento mientras esté usando ORENCIA.
Embarazo y lactancia
Se desconocen los efectos de ORENCIA en el embarazo, por lo tanto no debe utilizar ORENCIA si está usted embarazada a no ser que su médico se lo recomiende expresamente.
- si es usted una mujer que podría quedarse embarazada, debe utilizar métodos anticonceptivos fiables (control de natalidad) mientras esté usando ORENCIA y hasta 14 semanas después de la última dosis. Su médico le aconsejará sobre métodos idóneos.
- si usted se queda embarazada durante el tratamiento con ORENCIA, informe a su médico.
Si usted recibiera ORENCIA durante su embarazo, su bebé podría tener un riesgo más alto de contraer una infección. Es importante que usted informe a los médicos sobre su bebé y a otros profesionales sanitarios sobre el uso de ORENCIA durante su embarazo antes de que le administren cualquier vacuna (para más información ver sección sobre vacunación).
Se desconoce si ORENCIA pasa a la leche materna. Usted debe dejar de amamantarsi está siendo tratado con ORENCIA hasta 14 semanas después de la última dosis.
Conducción y uso de máquinas
No es de esperar que el uso de ORENCIA afecte a la capacidad para conducir, montar en bicicleta o usar máquinas. Sin embargo, si usted se siente cansado o indispuesto después de la administración de ORENCIA, no debe conducir, montar en bicicleta ni manejar ninguna maquinaria.
ORENCIA contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis, es decir, esencialmente "exento de sodio".
3. Cómo usar ORENCIA
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
ORENCIA solución inyectable se inyecta bajo la piel (vía subcutánea).
Dosis recomendada en adultos
La dosis recomendada de ORENCIA para adultos con artritis reumatoide o artritis psoriásica es de 125 mg cada semana independientemente del peso.
Su médico puede comenzar su tratamiento con ORENCIA con o sin una dosis única de polvo para concentrado para solución para perfusión (que se administra en la vena, por lo general en el brazo, durante un período de 30 minutos). Si se le administra una única dosis intravenosa para comenzar el tratamiento, la primera inyección subcutánea de ORENCIA se debe administrar en el plazo de un día desde la perfusión IV, seguidos de las inyecciones subcutáneas semanales de 125 mg.
ORENCIA se puede utilizar en adultos mayores de 65 años sin necesidad de cambiar la dosis.
Uso en niños y adolescentes
Para pacientes de 2 a 17 años con artritis idiopática juvenil poliarticular, la dosis semanal recomendada de ORENCIA solución inyectable en jeringa precargada, se basa en su peso corporal:
Dosis semanal de ORENCIA
Peso corporal del paciente | Dosis |
10 kg a menos de 25 kg | 50 mg |
25 kg a menos de 50 kg | 87,5 mg |
50 kg o más | 125 mg |
Si ya está recibiendo tratamiento con ORENCIA intravenoso y desea cambiar a ORENCIA subcutáneo, debe recibir una inyección subcutánea en lugar de su siguiente perfusión intravenosa, seguida por inyecciones subcutáneas semanales de ORENCIA.
Su médico le informará sobre la duración del tratamiento y qué otros medicamentos, incluyendo otros medicamentos modificadores de la enfermedad, si los hubiera, puede seguir tomando mientras esté en tratamiento con ORENCIA.
Al comienzo, su médico o enfermera puede inyectarle ORENCIA. Sin embargo, usted y su médico podrían decidir que puede inyectarse ORENCIA usted mismo. En este caso, se le instruirá acerca de cómo inyectarse ORENCIA usted mismo.
Consulte a su médico si tiene alguna duda acerca de la administración de la inyección. Encontrará instrucciones detalladas para la preparación y administración de ORENCIA al final de este prospecto (ver “Instrucciones importantes de uso”).
Si usa más ORENCIA del que debe
Si esto ocurre, póngase en contacto inmediatamente con su médico, que le vigilará por si tuviera algún signo o síntoma de padecer efectos adversos, y se los tratará si fuera necesario.
Si olvidó usar ORENCIA
Lleve un seguimiento de su siguiente dosis. Es muy importante usar ORENCIA exactamente como le indique su médico. Si usted se olvidó la dosis dentro del plazo de tres días respecto a cuando le correspondía, adminístrese la dosis en cuanto se acuerde y luego siga su pauta posológica original en el día elegido. Si se olvida de su dosis durante más de tres días, pregunte a su médico cuándo debe administrarse la próxima dosis.
Si interrumpe el tratamiento con ORENCIA
La decisión de interrumpir el tratamiento con ORENCIA debe ser comentada con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermera.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los efectos adversos más frecuentes que se producen con ORENCIA son infecciones de las vías aéreas superiores (incluidas las infecciones de nariz y garganta),dolor de cabeza y náuseas. ORENCIA puede producir efectos adversos graves que pueden requerir tratamiento.
Posibles efectos adversos gravesincluyen infecciones graves, neoplasias malignas (cáncer) y reacciones alérgicas, tal y como se enumeran a continuación.
Informe a su médico inmediatamentesi nota cualquiera de los siguientes síntomas:
- erupción grave, urticaria u otros síntomas de reacción alérgica
- cara, manos o pies hinchados
- dificultad para respirar o tragar
- fiebre, tos persistente, pérdida de peso, cansancio
Informe a su médico inmediatamentesi usted nota cualquiera de los siguientes:
- malestar general, problemas dentales, sensación de quemazón al orinar, erupción cutánea dolorosa, ampollas dolorosas en la piel, tos
Los síntomas descritos anteriormente pueden ser signos de los efectos adversos enumerados a continuación, los cuales se han observado con ORENCIA en ensayos clínicos en adultos:
Listado de efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes):
- infecciones de las vías respiratorias superiores (incluyendo infecciones de nariz, garganta y senos nasales).
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes):
- infecciones de los pulmones, infecciones urinarias, ampollas dolorosas en la piel (herpes), gripe
- dolor de cabeza, vértigo
- presión arterial elevada
- tos
- dolor abdominal, diarrea, náuseas, malestar de estómago, llagas en la boca, vómitos
- erupción cutánea
- fatiga, debilidad, reacciones en el lugar de la inyección
- pruebas de función hepática anormales
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes):
- infección dental, infección de uñas por hongos,infección muscular, infección del torrente sanguíneo, acumulación de pus bajo la piel, infección renal, infección de oído
- recuento bajo de leucocitos en sangre
- cáncer de piel, verrugas
- nivel bajo de plaquetas en sangre
- reacciones alérgicas
- depresión, ansiedad, alteración del sueño
- migrañas
- entumecimiento
- ojo seco, disminución de la visión
- inflamación de los ojos
- palpitación, ritmo cardíaco rápido, ritmo cardíaco lento
- presión arterial baja, sofocos, inflamación de los vasos sanguíneos, rubor
- dificultad para respirar, sibilancias, falta de aire, empeoramiento agudo de una enfermedad del pulmón llamada enfermedad pulmonar obstructiva crónica (EPOC)
- opresión de la garganta
- rinitis
- aumento de tendencia a la aparición de cardenales, piel seca, psoriasis, rojeces en la piel, sudoración excesiva, acné
- alopecia, prurito, urticaria
- articulaciones dolorosas
- dolor en las extremidades
- ausencia de menstruación, reglas abundantes
- síndrome pseudogripal, aumento de peso
Raros(pueden afectar hasta 1 de cada 1000 pacientes):
- tuberculosis
- inflamación del útero, trompas de Falopio y/o los ovarios
- infección gastrointestinal
- leucemia, cáncer de pulmón
Niños y adolescentes con artritis idiopática juvenil poliarticular
Los efectos adversos en niños y adolescentes con artritis idiopática juvenil articular son similares a los experimentados en adultos descritos anteriormente, con las siguientes diferencias:
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes):
- infección de las vías respiratorias superiores (incluidas infecciones de naríz, senos nasales y garganta)
- fiebre
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes):
- sangre en orina
- infección de oído
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ORENCIA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta después de EXP y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8°C). No congelar.
Conservar en el embalaje original para protegerlo de la luz.
No use este medicamento si el líquido está turbio o decolorado, o tiene partículas grandes. El líquido debe ser de incoloro a amarillo pálido.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ORENCIA
ORENCIA 50 mg solución inyectable en jeringa precargada
- El principio activo es abatacept.
- Cada jeringa precargada contiene 50 mg de abatacept en 0,4 ml.
ORENCIA 87,5 mg solución inyectable en jeringa precargada
- El principio activo es abatacept.
- Cada jeringa precargada contiene 87,5 mg de abatacept en 0,7 ml.
ORENCIA 125 mg solución inyectable en jeringa precargada
- El principio activo es abatacept.
- Cada jeringa precargada contiene 125 mg de abatacept en un ml.
- Los demás componentes son sacarosa, poloxamero 188, dihidrógeno fosfato sódico monohidrato, fosfato disódico anhidro y agua para preparaciones inyectables (ver sección 2 "ORENCIA contiene sodio").
Aspecto del producto y contenido del envase
ORENCIA solución inyectable (inyección) una solución transparente, de incolora a amarillo pálido.
ORENCIA está disponible en las siguientes presentaciones:
ORENCIA 50 mg solución inyectable en jeringa precargada con émbolo blanco
- envase de 4 jeringas precargadas con protector de aguja.
ORENCIA 87,5 mg solución inyectable en jeringa precargada con émbolo azul claro
- envase de 4 jeringas precargadas con protector de aguja.
ORENCIA 125 mg solución inyectable en jeringa precargada con émbolo naranja
- envases de 1 ó 4 jeringas precargadas y envase múltiple de 12 jeringas precargadas (3 envases de 4).
- envases de 1, 3 ó 4 jeringas precargadas con protector de aguja y envase múltiple de 12 jeringas precargadas con protector de aguja (3 envases de 4).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Bristol‑Myers Squibb Pharma EEIG
Plaza 254
Blanchardstown Corporate Park 2
Dublin 15, D15 T867
Irlanda
Responsable de la fabricación
CATALENT ANAGNI S.R.L.
Loc. Fontana del Ceraso snc
Strada Provinciale 12 Casilina, 41
03012 Anagni (FR)
Italia
Swords Laboratories Unlimited Company t/a Bristol‑Myers Squibb Cruiserath Biologics
Cruiserath Road, Mulhuddart
Dublin 15
Irlanda
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
Instrucciones importantes de uso. Leer detenidamente.
CÓMO USAR
ORENCIA 50 mg
ORENCIA 87,5 mg
ORENCIA 125 mg
solución inyectable en jeringa precargada con protector de aguja
Abatacept
Vía subcutánea
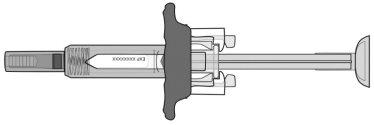
Lea estas instrucciones antes de usar ORENCIA jeringa precargada.
Antes de usar la jeringa precargada por primera vez, asegúrese de que su médico, enfermero o farmacéutico le haya mostrado la manera correcta de usarla.
Mantenga la jeringa precargada refrigerada hasta que esté lista para usar.NO CONGELAR.
Si tiene alguna pregunta sobre este producto, por favor lea el Prospecto.
ANTES DE EMPEZAR:
Conozca la jeringa precargada
Hay 3tiposde jeringas precargadas:
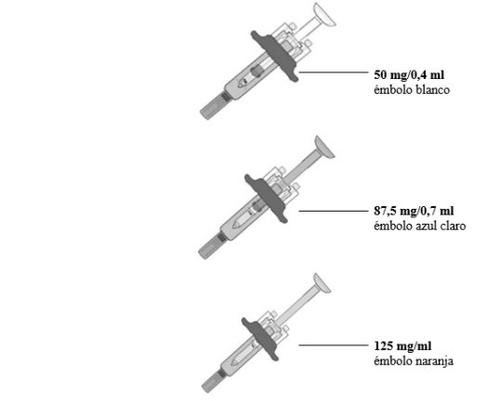
El tipo de jeringa precargada que se le administre depende de la dosis recetada por su médico. La jeringa precargada de 125mg/ml se muestra a continuación.
Antes de usar
Después de usar
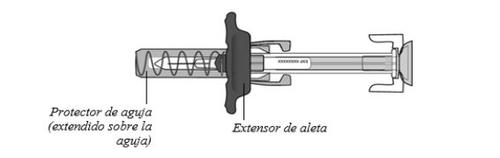
La jeringa precargada tiene un extensor de aletaque facilita la sujeción y la inyección, y un protector de agujaque de forma automática cubre la aguja después de finalizar la inyección.

NOquite la tapa de la aguja hasta que esté preparado para inyectar.
NO TIREdel émbolo en ningún momento.
NO VUELVA A TAPARla jeringa precargada en ningún momento, ya que esto puede dañar, doblar o romper la aguja.
Sujete siempre la jeringa por el cuerpo.
Proceda al Paso 1
Paso1: Preparación para la inyección de ORENCIA
Reúna todo lo necesario para su inyección sobre una superficie limpia y lisa.
En el envase solo se incluye la jeringa precargada:
- Gasa con alcohol

- Tirita
- Bola de algodón o gasa

- Jeringa precargada con protector de seguridad de aguja pasivo
- Contenedor para objetos punzantes
Deje que su jeringa precargada se atempere.
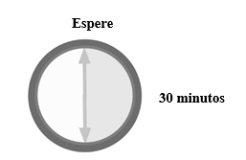
Saque una jeringa precargada de la nevera y espere 30minutospara que alcance la temperatura ambiente.
- Noacelere el proceso de atemperamiento de ningún modo, por ejemplo, usando el microondas o calentando la jeringa en agua tibia.
- Noquite la tapa de la aguja mientras deja que la jeringa precargada alcance la temperatura ambiente.
Lávese bien las manos con agua y jabón para prepararse para la inyección.
Proceda al Paso 2
Paso2: Examine la jeringa precargada
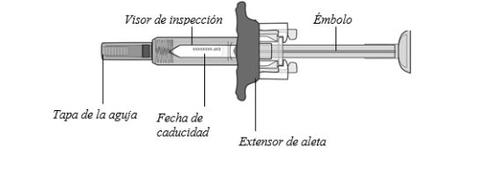
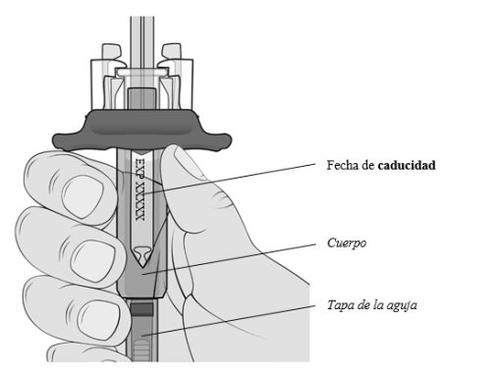
Sujete la jeringa precargada por el cuerpo con la tapa de la aguja apuntando hacia abajo como se muestra.
- Compruebe la fecha de caducidad impresa en la etiqueta.
Nola use si ha pasado la fecha de caducidad.
- Compruebe si la jeringa precargada está dañada.
Nola use si está agrietada o rota.
Comprobar el líquido
Comprobar el líquidoen la jeringa precargada a través del visor de inspección. Debe ser clara y de transparente a amarillo claro.
Puede que vea una burbuja de aire pequeña. Nointente eliminarla.
No se inyecte si el líquido está turbio, ha cambiado de color o tiene partículas visibles.
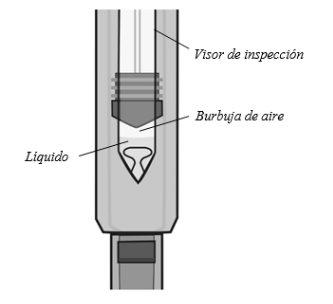
Nota: la figura muestra la jeringa precargada de 50 mg
Proceda al Paso 3
Paso3: Compruebe la dosis en la jeringa precargada
Sujete la jeringa a la altura de los ojos Mire de forma detenida para asegurarse que la cantidad de líquido en la jeringa precargada está o casi está por encima de la línea de llenadopara su dosis prescrita.
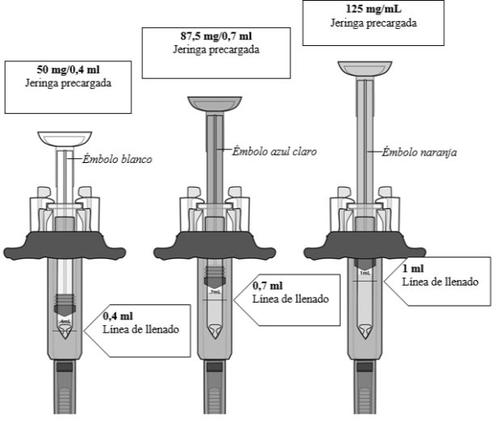
Nolo use si la jeringa precargada no tiene la cantidad correcta de líquido. Póngase en contacto con su médico, enfermero, o farmacéutico para más instrucciones.
Proceda al Paso 4
Paso4: Elija y prepare el lugar de inyección
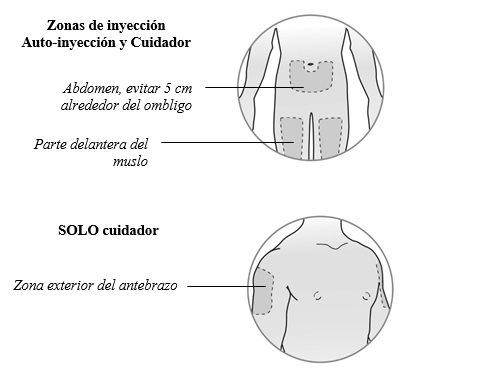
Elija el lugar de la inyecciónen el abdomen, parte anterior de los musloso la parte externa del antebrazo(solo si lo administra un cuidador).
Cambio del lugar de inyección
- Puede usar la misma zona del cuerpo cada semana, pero elija un lugar de inyección diferente en esa zona.
- Nose inyecte en áreas en las que la piel esté blanda, con cardenales, roja, descamada o dura.
Noadministre la inyección en ninguna zona con cicatrices o estrías.
- Registre la fecha, hora y lugar de inyección.
Limpie suavemente la zona de inyección
- Limpie suavemente la zona de inyección con una torunda de algodón y deje secar la piel.
- Notoque de nuevo el lugar de la inyección antes de administrar la inyección.
- Noabanique ni sople sobre el área limpia.
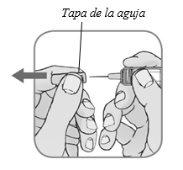
Retire la tapa de la agujasujetando el cuerpo de la jeringa precargada con una mano y tirando de la cubierta hacia fuera con su otra mano.
No ponga de nuevo la tapa de la aguja después de retirarla.Puede desechar la tapa en la basura doméstica después de la inyección.
- Nouse la jeringa precargada si gotea después de retirar la tapa de la aguja.
- Nouse la jeringa precargada si la aguja está dañada o doblada.
Nota: Es normal ver una gota de líquido saliendo de la aguja.
 NO VUELVA A TAPARla jeringa precargada, ya que esto puede dañar la aguja.
NO VUELVA A TAPARla jeringa precargada, ya que esto puede dañar la aguja.
Proceda al Paso 5
Paso5: Inyecte su dosis de ORENCIA
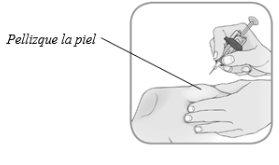
Sujete el cuerpode la jeringa precargada en su mano usando su dedo pulgar e índice. Con la otra mano, pellizque la zona de la piel que ha limpiado.
Inserte la aguja
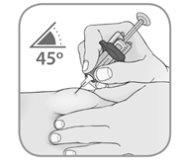
Inserte suavementela aguja en la piel pellizcada con un ángulo de 45º.
Complete TODOS los pasos para administrar su dosis completa de medicamento
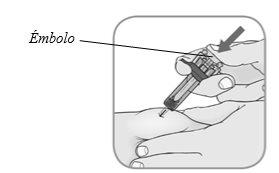
Inyecte: presione el émbolocon su pulgar hasta el final
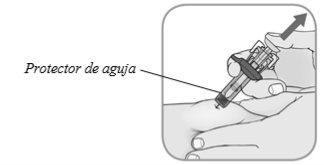
Quite el protector de aguja: levante lentamente su pulgar del émbolopara activar el protector de aguja.
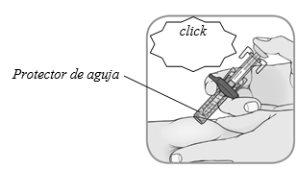
Confirmar:tras completar la inyección, el protector de aguja cubrirá la agujay oirá un click.
Retire la jeringa precargadadel lugar de la inyección y suelte la piel pellizcada.
Proceda al Paso 6
Paso6: Después de la inyección
Cuide el lugar de la inyección:
- Puede aparecer un leve sangrado en el lugar de la inyección. Puede presionar sobre el lugar de la inyección con una bola de algodón o una gasa.
- Nofrote el lugar de la inyección.
- Si es necesario, puede cubrir el lugar de la inyección con una tirita.
Deseche la jeringa precargada usadaen el contenedor para objetos punzantes inmediatamente después de usar. Si tiene alguna duda, pregunte a su farmacéutico.
Para más información sobre la eliminación, vea el Prospecto.
Si la inyección es administrada por un cuidador, esta persona debe manejar también con cuidado la jeringa para evitar lesiones accidentales por pinchazos con agujas y posible propagación de la infección.
Mantenga este medicamento y el contenedor para objetos punzantes fuera de la vista y del alcance de los niños.
Instrucciones importantes de uso
Lea cuidadosamente estas instrucciones y sígalas paso a paso.
Recibirá instrucciones de su médico o enfermera acerca de cómo autoinyectarse ORENCIA usando la jeringa precargada.
No intente autoinyectarse hasta que esté seguro de que entiende cómo preparar y administrar la inyección. Después de una formación adecuada, puede administrarse la inyección o se la puede administrar otra persona, por ejemplo, un miembro de la familia o un amigo.
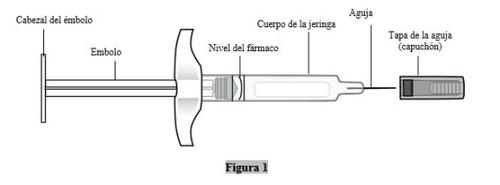
Antes de empezar–cosas que deben hacerse y no hacerse
Hacer
- Manipule siempre la jeringa de ORENCIA cuidadosamente, especialmente cuando esté junto a otras personas o cerca de niños.
- Sujete siempre la jeringa por el cuerpo.
- Conserve las jeringas no utilizadas en la nevera en la caja original.
- Tenga preparados los suministros de inyección adicionales antes de inyectarse.
- Lista de comprobación de suministros:torundas de alcohol, bola de algodón o gasa, tirita, contenedor de objetos punzantes.
Los contenedores de objetos punzantes son cubos de eliminación resistentes a la punción que pueden comprarse en muchas tiendas.
No hacer
- Noretire el protector de la aguja (capuchón) hasta que esté listo para inyectar.
- Notire del émbolo hacia atrás en ningún momento.
- Noagite la jeringa, porque esto puede dañar el medicamento ORENCIA.
- NOvuelva a poner el capuchón de la aguja.
PASO1: Tenga la jeringa preparada
- Compruebe la fecha de caducidad y el número de lote en la caja
- La fecha de caducidad puede encontrarse en la caja de ORENCIA y en cada jeringa.
- Si la fecha de caducidad ha pasado, no utilice las jeringas. Póngase en contacto con su médico o farmacéutico si necesita ayuda.
- Deje que la jeringa se caliente
- Encuentre un lugar cómodo con una superficie de trabajo limpia y plana.
- Extraiga la jeringa de la nevera. Mantenga cualquier jeringa restante no utilizada en su caja original, en la nevera.
- Compruebe que la fecha de caducidad y el número de lote se corresponden con los de la caja.
- Inspeccione la jeringa por si tiene defectos obvios, pero no retire la tapa de la aguja.
- Deje que la jeringa repose a temperatura ambiente durante 30 a 60 minutos antes de inyectarse.
- Noacelere el proceso de calentamiento de ningún modo, por ejemplo, usando el microondas o calentando la jeringa en agua tibia.
- Compruebe el líquido en la jeringa
- Sujete la jeringa por el cuerpo, con la aguja cubierta apuntando hacia abajo.
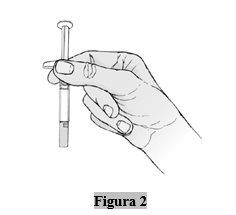
- Mire el líquido en la jeringa (Figura 2). El líquido debe ser de transparente a amarillo pálido.
- Nola inyecte si el líquido está turbio o ha cambiado de color o tiene partículas visibles.
- Es normal ver una burbuja de aire y no hay razón para retirarla. Debe inyectarse todo el contenido de la jeringa.
- Reúna sus suministros adicionales y manténgalos a su alcance.
- Lávese las manos concienzudamente con agua tibia y jabón.
PASO2: Elija y prepare el lugar de la inyección
Tenga la jeringa lista para su uso inmediatamente después de haber preparado el lugar de la inyección.
- Elija una zona del cuerpo para la inyección (lugar de inyección)
- Puede utilizar:
- La parte anterior del muslo
- El abdomen, excepto el área de 5 cm alrededor del ombligo (Figura 3).
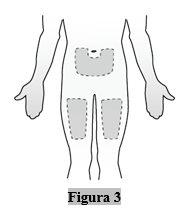
- Elija un lugar de inyección diferente para cada nueva inyección. Puede usar el mismo muslo para las inyecciones semanales, siempre que cada lugar de inyección esté separado aproximadamente 2,5 cm del lugar donde se inyectó por última vez.
- Nose inyecte en áreas en las que la piel esté dolorida, con hematomas, roja, descamada o dura. Evite las zonas con cicatrices o estrías.
- Prepare el lugar de inyección
- Limpie el lugar de inyección con una torunda con alcohol mediante un movimiento circular.
- Deje secar la piel antes de inyectar.
- Notoque de nuevo el lugar de la inyección antes de administrar la inyección.
- Noabanique ni sople sobre el área limpia.
PASO3: Inyectar ORENCIA
- Retire la tapa de la aguja (capuchón) sólo cuando esté preparado para administrarse la inyección.
- Sujete la jeringa por el cuerpo con una mano y tire de la tapa de la aguja directamente con la otra mano (Figura 4).
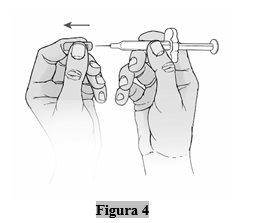
Puede haber una pequeña burbuja de aire en el líquido de la jeringa. No es necesario eliminar la burbuja de aire.
Puede apreciar una gota de líquido que sale de la aguja. Esto es normal y no afectará a la dosis.
- Notoque el émbolo mientras retira la tapa de la aguja.
- No retire la tapa de la aguja hasta que esté listo para inyectar ORENCIA.
- Notoque la aguja ni deje que toque ninguna superficie.
- Noutilice la jeringa si se cae sin llevar colocada la tapa de la aguja.
- Novuelva a poner la tapa de la aguja en la aguja una vez retirada.
- Noutilice la jeringa si hay signos visibles de que la aguja esté dañada o torcida.
- Coloque la jeringa e inyecte ORENCIA
- Sujete la jeringa por el cuerpo en una mano entre el dedo pulgar y el índice (Figura 5).
- Nopresione en la cabeza del émbolo hasta que comience la inyección.
- Notire hacia atrás del émbolo en ningún momento.
- Usando la otra mano, pellizque suavemente la zona de la piel que ha limpiado. Sujete firmemente.
- Inserte la aguja con un movimiento rápido en la piel pellizcada con un ángulo de 45º (Figura 5).
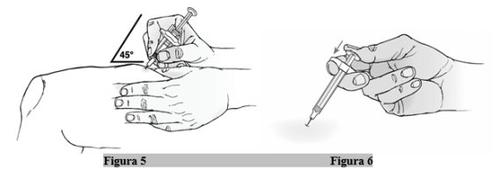
- Utilice el pulgar para empujar el émbolo hacia abajo, presionando firmemente hasta que el émbolo ya no avance más y se haya inyectado todo el medicamento (Figura 6).
- Retire la aguja de la piel y suelte la piel circundante.
- Novuelva a tapar la aguja.
- Presione con una bola de algodón sobre el lugar de inyección y mantenga la presión durante 10 segundos.
- Nofrote el lugar de la inyección. Es normal un sangrado leve.
- Si es necesario, puede aplicar una pequeña tirita en el lugar de la inyección.
PASO4: Eliminación de la jeringa y registro
- Deseche la jeringa usada en un contenedor de objetos punzantes.
- Pregunte a su médico, enfermera o farmacéutico acerca de las leyes nacionales y locales sobre la eliminación adecuada de productos médicos que contienen agujas.
- Mantenga siempresu contenedor de objetos punzantes fuera del alcance de los niños y los animales.
- Notire las jeringas usadas en la basura doméstica o en cubos de reciclado.
- Lleve un registro de la inyección
- Escriba la fecha, la hora y la parte concreta del cuerpo donde se ha inyectado. También puede ser útil escribir cualquier pregunta o preocupación sobre la inyección, de modo que pueda preguntar a su médico, enfermera o farmacéutico.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ORENCIA 125 mg SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, Cada pluma precargada contiene 125 mg de abatacept en un mlPrincipio activo: AbataceptFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: INYECTABLE PERFUSIONPrincipio activo: AbataceptFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: COMPRIMIDO, 180 mgPrincipio activo: ácido micofenólicoFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para ORENCIA 125 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ORENCIA 125 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






